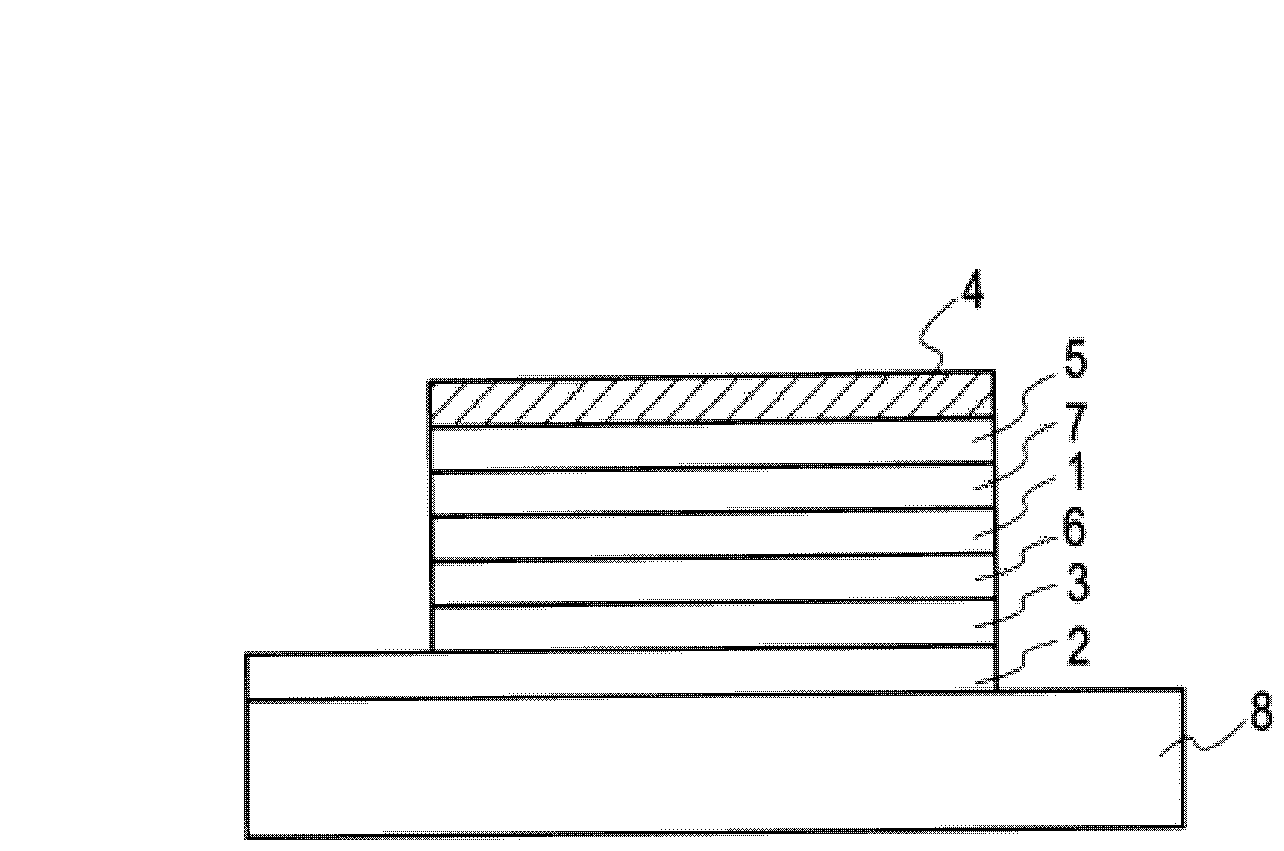
유기 일렉트로닉스용 재료, 유기 일렉트로닉스 소자, 유기 일렉트로 루미네센스 소자, 및 그것을 사용한 표시 소자, 조명 장치, 표시 장치
The present invention refers to, organic (organic) material for electronics, and said organic electronic material using organic electronic device, organic electroluminescence element (hereinafter, referred to as organic EL display element), and using the same display device, lighting device, a display device are disclosed. Organic electronic element, and the organic solution of a memory operations, energy saving, cost, flexibility advantages such as taste in the can be produced, which mainly consists of silicon of the existing method can replace the inorganic (inorganization) semiconductor wherein as a technique in the spotlight disclosed. Organic EL element among organic electronic device, for example, incandescent lamp, gas filling lamp as replacement, large area ([...]) solid state (solid-a state) use as light source in the spotlight disclosed. In addition, flat panel display (FPD) liquid crystal display (LCD) in the field to replace the spotlight to allow the most potent self-luminous display programs, reduced number article progressing disclosed. Organic EL element, a low-molecular type organic EL element using material and number film (or less and) according to the method, polymer type organic EL for 2 types distinguished by other. Polymer type organic EL element, organic material is a high molecular material is used, forming a low molecular weight type organic EL required in gauge in the device, such as inkjet printing or capable of forming a simple because, large-scaled ([...]) organic EL display device is indispensable for future usage times are disclosed. Low molecular weight type organic EL element, polymer type organic EL element, but has to be extended until now study is performed, still low luminous efficiency, short a handset door number element life point in the nanometer range. In order to solve this door number point as one means, low molecular weight type organic EL multilayer elements by means disclosed. One example of a multi-layer organic EL 1 also exhibits. In fig. 1, light emitting eletrolysis emitting layer (1), provided with other layer, anode (2) hole injection layer in contact with the layer (3), cathode (4) layer in contact with the metal layer (5) (description) is described as follows. In addition, light emitting layer (1) and hole injection layer (3) in the presence of between other layers, hole transport layer (6) and describes, in addition light emitting layer (1) and the metal layer (5) in the presence of between other layers, an electron transport layer (7) is described. And, in also 1, code "8" substrate are disclosed. The number molecular weight type organic EL element performing the substrate film, sequentially changed while the radiation is deposition compounds using multilayer can be achieve by performing for hereinafter. On the other hand, polymer type organic EL element such as inkjet printing or wet process (number (wet process) is performed using film, multilayer in order to, new layer layers need not changed it closes but it will do just now number number method disclosed. Polymer type organic EL element since the object for fitting in multilayer, contact is lower (lower stratum), is formed on the upper layer (upper layer) dissolved with each other's oldest. The aforementioned door to the processing number, been made regarding the solubility greatly different compounds. The exemplary embodiment, film polythiophenes performed using aqueous dispersion (wednesday dispersion) number: polystyrene (PEDOT: PSS) the mote [leyn[leyn] the opinion phone it buys consisting of a hole injection layer, using a solvent-based organic aromatic such as toluene number 2 is connected to the main element performing film layer removed is cited. In this case, PEDOT: PSS layer is insoluble in the toluene layer structure is number 2 bath has been possible. However, it is difficult to stand-alone water number, organic electronic device characteristics of causes degradation in with each other. In addition number for a stand-alone water, high temperature, long-term drying is necessary and, resin base material ([...]) high pressure liquid coolant or difficult on organic electronic element number, process pressure condition caused large number one airway as follows. In addition, organic solvent in one example, first forming a lower layer of a solvent incapable of affecting the disclosure in the nanometer range (patent document 1 reference) selecting method. However, the method can be applied to a point where the solvent is defined which is incapable of dissolving a lower since, material selection exists in the number whose width is flow tides. In addition, some degree of lower erosion of the upper is formed causing substrate. Other multilayer structures as a method, the method using a cross-reactive disclosure and the second region. Patent document 2 is, triphenyl amine-containing ether to polyether ketone by UV radiation, which method is insoluble ingredients disclosure in the nanometer range. In the method, in order to sufficiently electric arc furnace dust, long-term ultraviolet irradiation direction is, if it is assumed that decomposition of triphenylamine door number of flow tides. In addition, patent document 3, patent document 4, non-patent document 1, non-patent document 2 is, oxetane particle size of multilayer by disclosure and the second region. In these method, using optical disclosure number since, by Faraday tunneling effect with each other. In addition, sufficient solubility at low temperature is not in progress, the number and the first door number one need application of low temperature cure resin substrate formed organic EL or upper side are mixed door number of property is flow tides. In addition, an optical disclosure number is used for this, or sulfonyl [nyum salt is general five degree [nyum salt, EL characteristics influence on a tunneling effect substrate. On the other hand, deterioration of organic EL for and in driving voltage for number, five degree [nyum salt having hole transporting layer or light emitting layer is the same in structure or sulfonyl [nyum salt using review is not disclosed. Patent document 5 is, ion compound but the disclosure, this disclosure number said light passage to the same, the adverse influence of the organic EL element characteristics is a tunneling effect substrate. In addition, cross-linking is described with respect to the stack or not disclosed. 6 nucleotide is patent, polymer containing polymer illuminant composition for ion pair together disclosure in the nanometer range. In this way, a multilayer structure of ion pair in that long life by having photoresist pattern changed described but, charge injection, transport for the described not disclosed. In addition, cross-linking can be monitored with respect to the stack or not disclosed. On the other hand, to improve the efficiency of organic EL, phosphorescent organic EL element also development of extended performed disclosed. Organic EL phosphor elements, of the energy of a singlet state (singlet state) using energy as well as triplet state (triplet state) is possible, it can be 100% internal quantum yield principle to the height. Organic EL phosphor elements, as impurities including heavy metals such as platinum or iridium metal tightly the system emitting phosphor luminescent material, host material by doping a phosphorescent light-emitting against other (non-patent document 3, non-patent document 4, non-patent document 5 reference). The patent document 7, organic EL phosphor multilayer by polymerizing the polymerizable compound of the device disclosed. The adjacent layer that is a light-emitting layer is in an disclosure number, are disclosure number polymerization or decomposition, the emitting layer by one of the reactive compounds, organic EL for which shorten the service life as a probe substrate. Therefore, light emitting layer the adjacent layers on a number was not a structure does not contain disclosure number. However, the adjacent layers on a light emitting layer comprising said number in the within disclosure number avoid, polymer layer including a light emitting layer will not cause disclosure number adjacent to, and on a lower disclosure number containing, polymerizable compound to cure the layer including an upper layer, and also with the choice of the incapable of dissolving an error due to a lower upper, the material can be used pin is number one. In addition, an error due to upper and lower layers constituting the upper layer, the upper layer is incorporated in the polymer disclosure number, number of short life under each door pin is point. The present invention refers to, and then the aforementioned door number, driving voltage reduction or even in long time stable organic electronic element number reference voltage can be a high pressure liquid coolant under public affairs number intended for organic electronic material. In addition, the present invention refers to, coating technique using the same organic electronic material is curable with pipes and multilayer organic electronic element intended for the number under public affairs. In addition, the present invention refers to, a luminescent than conventional, with organic EL element life, using the same display device, lighting device, and display device intended for a under public affairs the number. The victims of the present invention, a kind review result, or sulfonyl [nyum[nyum]five degree [nyum[nyum] not used in general optical disclosure number, having particular relative cation (counter cations) charge transport compound by including an ionic compound, which can solve the aforementioned and number and thus, the present invention has been completed. In addition, the present invention the parties, more relevant result, multilayer structure 2 of two adjacent layer, each organic EL element substituent polymerizable compounds including high pressure liquid coolant mixture by number, organic EL emitting efficiency, life can be improved and thus, the present invention has been completed. I.e., the present invention refers to, for (1) and (38) of these alterations of ∼ characterized. (1) at least, ion compound and charge-transporting units compounds (hereinafter, charge-transporting compound soap without) as containing organic electronic material, Said ion compound, relative relative anion (counter anions) made of silica, said relative cation, H+ , Carbon cation, cationic nitrogen, oxygen cation, and transition metal cation having either at least 1 species or 2 species characterized organic electronic material. (2) carbon cation 3 characterized in that the class carbon cation (1) a organic electronic material. (3) nitrogen cation, characterized in that said a class number 3 or number 4 class nitrogen cation (1) or said (2) a organic electronic material. (4) relative anion, fluoro phosphate ion current, fluorinated alkyl fluoro phosphate ion current, boric acid ion current, and a fluoro group either antimony acid ion current is not less than 1 species or 2 species characterized, said (1) ∼ (3) at least one of organic electronic material described. (5) charge-transporting compound, triarylamine, carbazole, and thiophenes and at least 1 having a structure selected from said at least one (1) (4) ∼ described at least one of organic electronic material. (6) charge-transporting compound, by the general formula (1a) having a repeating unit represented by the hole transport property ∼ (7a) characterized in that said minute particles including a polymer or oligomer (1) (4) ∼ described at least one of organic electronic material. [Formula 1] (A coloring composition, Ar1 ∼Ar20 Is, independently aryl group or a heteroaryl group of 2 ∼ 30 carbon atoms, or a substituted or nonsubstituted it will be biting, [leyn, it will be biting and [leyn it will raise hetero exhibits. Wherein, the aryl is a stand-alone number 1 hydrogen atom from aromatic hydrocarbons dog a donating group (atomic group), hetero aryl, hetero atoms in the aromatic compound having a hydrogen atom from a dog donating group number 1 a stand-alone, or R by a goniophotometer. R - R are each independently1 , - OR2 , SR3 , - OCOR4 , - COOR5 , - SiR6 R7 R8 Or general formula (2a) ∼ (4a) (stage, R1 ∼R8 Is, hydrogen atom, 1 ∼ 22 carbon atoms of linear, cyclic or branched alkyl (annulation), aryl group or heteroaryl group of carbon number 2 ∼ 30 display) exhibits. Wherein, a dog and a stand-alone hydrogen atom donating group number 2 it will be biting and [leyn it crawls from aromatic hydrocarbons, heterocyclic it will be biting and [leyn it crawls, an aromatic compound having a hydrogen atom from a donating group number 2 heteroatoms dog stand-alone are disclosed. X is during said R, having one or more hydrogen atoms from group 1, a stand-alone hydrogen atoms number 1 further groups of phenoxyethanol.) (7) in addition, charge-transporting compound, characterized in that said one or more polymerizable substituents 1 fixes the position of a (1) (6) ∼ described at least one of organic electronic material. (8) polymerizable substituents, oxetane, epoxy, and vinyl ether characterized in that said at least one least one (7) a organic electronic material. (9) characterized in that said further including a solvent (1) (8) ∼ described at least one of organic electronic material. (10) ion compound and an electron-accepting compound, charge-transporting compounds can be characterized in that the electronic oxidation (one a-electron oxidation) 1 (1) (9) ∼ described at least one of organic electronic material. (11) said (1) ∼ (10) described in one of the organic electronic material, and is applied onto a substrate, characterized in that chemical vapor deposition layer the organic electronic device. (12) characterized in that said chemical vapor deposition a layer electric arc furnace dust (11) a organic electronic device. (13) on solubility, more deposition and, characterized in that said multiple layers in terms a (12) a organic electronic device. (14) substrate, characterized in that the resin film (11) ∼ (13) at least one a organic electronic device. (15) said (1) (10) ∼ described at least one of organic electronic material characterized with a layer formed the organic electroluminescence element. (16) at least substrate, anode, hole injection layer, polymer layer, each comprising a light emitting layer and a cathode as organic electro-luminescence element, said polymer layer, said (1) ∼ (10) is formed by at least one of organic electronic material described organic electroluminescence element. (17) at least substrate, anode, polymer layer, hole transport layer, each comprising a light emitting layer and a cathode as organic electro-luminescence element, said polymer layer, said (1) ∼ (10) is formed by at least one of organic electronic material described organic electroluminescence element. (18) a white luminescent color of organic electro-luminescence element characterized in that said (15) (17) ∼ described at least one of the organic electroluminescence element. (19) is composed of a multilayer structure, adjacent 2 dog layer of said multilayer structure, the hole-transporting compound including each substituent polymerizable mixture is formed by coating technique and, each said hole-transporting organic electro characterized by formula consists of a light emitting element. (20) said polymerizable substituents, epoxy, oxetane, and said at least one or more of vinyl ether (19) a organic electro-light-emitting device. (21) said hole transporting compound, at least arylamine, carbazole, including said thiophene skeleton (19) or said (20) a organic electro-light-emitting device. (22) said hole transporting compound, number average molecular weight of 1000 or more oligomer or polymer said (19) (21) ∼ a at least one organic electro-light-emitting device. (23) adjacent said during wustite 2, anode layer near said mixed with only polymerization disclosure number (19) ∼ (22) at least one organic electro-a light emitting element. (24) said polymerization disclosure number is, carbon cation, cationic nitrogen, oxygen cation, and selected from the group consisting of transition metal cations having radially inward and is cation ion compound having said (23) a organic electro-light-emitting device. (25) adjacent said dog layer 2, hole injection layer layer, said light emitting layer formed on the hole transporting layer (19) ∼ (24) at least one organic electro-a light emitting element. (26) including said said light emitting layer is a metal complex (bodies) (25) a organic electro-light-emitting device. (27) said ion compound and an electron-accepting compound, said hole transport compound 1 can be characterized in that said electronic oxidized (24) ∼ (26) at least one organic electro-a light emitting element. (28) said organic electro-light-emitting device substrate, said a resin film (19) ∼ (27) a at least one organic electro-light-emitting device. (29) a white luminescent color of organic electro-luminescence element characterized in that said (16) (18) ∼ either or said (19) ∼ (28) described in one of the organic electro-light-emitting device. (30) the substrate of the organic electro-luminescence element, characterized in that said flexible substrate a (16) (18) ∼ either or said (19) ∼ (27) described in one of the organic electro-light-emitting device. (31) said (15) (20) ∼ either or said (19) ∼ (30) described in one of the organic electro-light emitting element display element. (32) said (15) (20) ∼ either or said (19) ∼ (30) a illumination device either organic electro-light emitting element. (33) said (32) on a illumination device, liquid crystal display device the display element. The disclosure of the present disclosure, application Japan to the Japanese patent application number 2009 - 229483 1 October 2009 to the Japan application 14 January 2010 and a Japanese patent application number 2010 - 5846 which associated with a main number, the contents of their disclosure citation thereto by staff with each other. According to the present invention, reducing a drive voltage of an organic electronic element number reference voltage over a prolonged period can be high pressure liquid coolant, in a relatively low temperature coating technique is curable organic electronic material, using the same multilayer organic electronic device, organic electroluminescence element, display device and lighting device can be a number under public affairs. I.e., by containing an ionic charge transport compound, organic electronic element reducing driving voltage reference voltage over a prolonged period, in particular organic EL element is formed. In addition, according to the present invention, coating technique can be achieved and by multilayered organic EL element, a luminescent or with organic EL element can be life number under public affairs, uses it to its properties display device, lighting device, and display device can be a number under public affairs. Figure 1 shows a multilayer organic EL indicating one example of a mimetic also are disclosed. <Organic electronic material> Organic electronic material of the present invention, at least, ion compound and charge-transporting units compounds (hereinafter, charge-transporting compound soap without) as containing organic electronic material, said ion compounds, silica made relative relative anion, said relative cation, H+ , Carbon cation, cationic nitrogen, oxygen cation, transition metal cation having either at least 1 species or 2 species characterized. In hereinafter, each component of the organic electronic material detailed of the present invention are described as follows. [Ion compound] Apparatus of compounds in the present invention, the aforementioned relative cation and mating with the anion combustion chamber. And, relative cations are present, H+ , Carbon cation, cationic nitrogen, oxygen cation, transition metal cation is at least one species having either 1 or 2. Hereinafter, each cations are disclosed therein. (Carbon cation) As carbon cation, class number 1 carbon cation, carbon cation class number 2, number 3 class carbon cation are exemplified. In one of the, class number 2 carbon cation, anion carry when stability of carbon cation material in combination with class number 3, is cured at a low temperature is preferably the polymerisable disclosure number, most preferably carbon cation class number 3. In addition, tree phenyl car [lu[lu] step [nyum[nyum] cation, tree (methylphenyl) carboxylic step [nyum[nyum] cation, tree (dimethylphenyl) carboxylic step [nyum[nyum] productivity are cation can be. (Nitrogen cation) As NH nitrogen cation4+ , Class number 1 cationic nitrogen, nitrogen cation class number 2, number 3 class nitrogen cation, class number 4 nitrogen cation are exemplified. Wherein, nitrogen cations are present N class number 1+ Two hydrogen atoms combined with 3, associated with a combination of other mm for matching with the other than hydrogen atom, nitrogen cations are present N class number 2+ 2 is combined with two hydrogen atoms, a combination of other mm for matching with the associated with the atoms other than hydrogen, nitrogen cations are present N class number 3+ 1 is combined with two hydrogen atoms, a combination of other mm for matching with the associated with the atoms other than hydrogen, quaternary ammonium cations are present N number 4+ Other than compounds is hydrogen atom are bonded by a goniophotometer. Specifically, n - butyl ammonium, dimethyl ammonium, trimethyl ammonium, tri-ethyl ammonium, tree isopropyl ammonium, tree - n - butyl ammonium, tetramethyl ammonium, tetraethyl orthosilicate ammonium, tetra - n - butyl ammonium, N, N - dimethyl hour claw [heyk thread ammonium, tetramethyl ammonium, ethyl tree methyl ammonium, d ethyl D methyl ammonium, tri-ethyl methyl ammonium, tetra ethyl ammonium, trimethyl - n - propyl ammonium, trimethyl isopropyl ammonium, trimethyl - n - butyl ammonium, trimethyl isobutyl ammonium, trimethyl - tert - butyl ammonium, trimethyl - n - [heyk[heyk] Thread ammonium, dimethyl di - n - propyl ammonium, dimethyl dee small pro the ammonium which will bloom, dimethyl - n - propyl isopropyl ammonium, methyl tree - n - propyl ammonium, ammonium such as for example methyl tree cow pro the ammonium which will bloom is cited. In addition, will not be [nyum methyl N -, N, N - dimethyl will not be [nyum, N, N - dimethyl - 4 - methyl will not be [nyum, N, it will not be a d ethyl [nyum N -, N, it will not be a d phenyl [nyum N -, N, N, N - tree such as it will not be a methyl [nyum will not be [nyum example is cited. In addition, [...] pyridazinone, N - methyl blood [tyum[tyum], N - butyl blood [tyum[tyum], N - methyl - 4 - methyl blood [tyum[tyum], blood [tyum[tyum] N - benzyl, 3 - methyl - N - butyl blood [tyum[tyum], blood [tyum[tyum] 2 - methyl, 3 - methyl blood [tyum[tyum], blood [tyum[tyum] methyl 4 -, 2, 3 - dimethyl blood [tyum[tyum], 2, 4 - dimethyl blood [tyum[tyum], 2, 6 - dimethyl blood [tyum[tyum], 3, 4 - dimethyl blood [tyum[tyum], 3, 5 - dimethyl blood [tyum[tyum], 2, 4, 6 - tri methyl blood [tyum, 2 - fluoro blood [tyum[tyum], blood [tyum[tyum] 3 - fluoro, 4 - fluoro blood [tyum[tyum], 2, 6 - HET transducer base five lithium, 2, 3, 4, 5, 6 - pen hit [phul base oro blood [tyum, 2 - chloro blood [tyum[tyum], blood [tyum[tyum] 3 - chloro, 4 - chloro blood [tyum[tyum], 2, 3 - dichloro blood [tyum[tyum], 2, 5 - dichloro blood [tyum[tyum], 2, 6 - dichloro blood [tyum[tyum], 3, 5 - dichloro blood [tyum[tyum], 3, 5 - dichloro - 2, 4, 6 - HET triple base five lithium, lithium 2 - cross fur, electricity fur lithium 3 -, 4 - cross fur lithium, with d [pu fur [tyum 2, 5 -, 2, 6 - with d [pu fur [tyum, with d [pu fur [tyum 3, 5 -, 2 - cyano roh blood [tyum[tyum], roh blood [tyum[tyum] 3 - cyano, 4 - cyano roh blood [tyum[tyum], hour blood [tyum[tyum] 2 - hydroxy, 3 - hydroxy hour blood [tyum[tyum], 4 - hydroxy hour blood [tyum[tyum], 2, 3 - d high [tu rock hour blood [tyum, 2, 4 - d high [tu rock hour blood [tyum, 2 - methyl - 5 - ethyl blood [tyum[tyum], 2 - chloro - 3 - cyano roh blood [tyum[tyum], 4 - carboxylic copy american evasion [tyum, the diplopia it knows, [tu blood [tyum 4 - carboxylic acid, 2 - phenyl blood [tyum[tyum], 3 - phenyl blood [tyum[tyum], blood [tyum[tyum] 4 - phenyl, 2, 6 - d phenyl blood [tyum, 4 - knit with blood [tyum, 4 - maul [thok hour blood [tyum, 4 - vinyl blood [tyum[tyum], 4 - [me[me] cop toe blood [tyum[tyum], 4 a-tert - butyl blood [tyum[tyum], 2, 6 - di - tert - butyl blood [tyum[tyum], 2 - benzyl blood [tyum[tyum], blood [tyum[tyum] acetyl 3 -, 4 - ethyl blood [tyum[tyum], the diplopia it buys blood [tyum 2 - carboxylic acid, 4 - carboxylic the diplopia it buys blood [tyum, such as for example benzoyl 2 - pyridyl blood [tyum[tyum][tyum[tyum] is cited. In addition, imidazolium, 1 - methyl - imidazolium, 1 - ethyl - 3 - methyl imidazolium, 1 - propyl - 3 - methyl already will be and the loom which will doze, 1 - butyl - 3 - methyl already will be and the loom which will doze, 1 - hexyl - 3 - methyl already will be and the loom which will doze, 1 - methyl - 3 - the jade [thil already will be and the loom which will doze, 1 - methyl - N - benzyl already will be and the loom which will doze, 1 - methyl - 3 - (3 - phenylpropyl) imidazolium, 1 - butyl - 2, 3 - dimethyl already will be and the loom which will doze, 1 - ethyl like example already will be and the loom which will doze - 2, 3 - dimethyl imidazole loom is cited. In addition, 1 - ethyl - 1 - methyl pyrrolidone lithium, 1 - butyl - 1 - methyl pyrrolidone lithium such as pyrrole lithium for example is cited. In addition, [khwi[khwi] glow [nyum[nyum], such as for example isocyanate [khwi[khwi] glow [nyum[nyum][khwi[khwi] glow [nyum[nyum] is cited. And, N, N - dimethyl pyrrole lithium, lithium N - ethyl - N - methyl pyrrolidone, N, N - d ethyl blood raleigh [tyum pyrrole such as for example lithium is cited. In addition, station number disclosure number 03/005076 call, station number disclosure number 03/097580 described diimmonium, [nyum[nyum] can process for example. Among, class number 3 nitrogen cation, class number 4 nitrogen cation, anion when carry in combination with stability, the disclosure number is preferably capable of cured at a low temperature, most preferably nitrogen cation class number 3. (Oxygen cation) Oxygen cation as, methyl iodine [nyum tree, tree [thil[thil] Iodine [nyum[nyum], tree pro the iodine which will bloom [nyum, butyl iodine [nyum tree, tree [heyk[heyk] Thread iodine [nyum[nyum], tree phenyl iodine [nyum, blood reel loom, compress maul neel loom (chromenylium), the [khu it buys [thil the loom can be (xanthylium) is exemplified. (Transition metal cation having) As the transition metal cation having, for example, Cr (η 5 - cyclopentadienyl) (η 6 - toluene)+ , Cr (η 5 - cyclopentadienyl) (η 6 - xylene)+ , Cr (η 5 - cyclopentadienyl) (η 6 - 1 - methyl naphthalene)+ , Cr (η 6 - cumene) (η 5 - cyclopentadienyl)+ , Cr (η 5 - cyclopentadienyl) (η 6 - mesh [thil)+ , Cr (η 6 - pyrene) (η 5 - cyclopentadienyl)+ , Cr (η 6 - cumene) (η 5 - fluorenyl)+ , Cr (η 6 - cumene) (η 5 - indenyl)+ , Bis (η 6 - mesh [thil) Cr2+ , Bis (η 6 - xylene) Cr2+ , Bis (η 6 - cumene) Cr2+ , Bis (η 6 - toluene) Cr2+ , Cr (η 6 - toluene) (η 6 - xylene)2 + , Cr (η 6 - cumene) (η 6 - naphthalene)2+ , Bis (η 5 - cyclopentadienyl) Cr+ , Bis (η 5 - indenyl) Cr+ , Cr (η 5 - cyclopentadienyl) (η 5 - fluorenyl)+ And (η 5 - cyclopentadienyl) Cr (η 5 - indenyl)+ Cr compound such as, in addition (η 5 - cyclopentadienyl) Fe (η 6 - toluene)+ , (Η 5 - cyclopentadienyl) Fe (η 6 - xylene)+ , (Η 5 - cyclopentadienyl) Fe (η 6 - 1 - methyl naphthalene)+ , (Η 5 - cyclopentadienyl) Fe (η 6 - cumene)+ , (Η 5 - cyclopentadienyl) Fe (η 6 - mesh [thil)+ , (Η 5 - cyclopentadienyl) Fe (η 6 - pyrene)+ , (Η 6 - cumene) Fe (η 5 - fluorenyl)+ , (Η 6 - cumene) Fe (η 5 - indenyl)+ , Bis (η 6 - mesh [thil) Fe2+ , Bis (η 6 - xylene) Fe2+ , Bis (η 6 - cumene) Fe2+ , Bis (η 6 - toluene) Fe2+ , (Η 6 - toluene) Fe (η 6 - xylene)2 + , (Η 6 - cumene) Fe (η 6 - naphthalene)2+ , Bis (η 5 - cyclopentadienyl) Fe2+ , Bis (η 5 - indenyl) Fe+ , (Η 5 - cyclopentadienyl) Fe (η 5 - fluorenyl)+ And (η 5 - cyclopentadienyl) Fe (η 5 - indenyl)+ Such as Fe compounds disclosed. (Relative anion) For use in the present invention relative anion is described substrate. As the anion, and if anything you conventional publicly known anionic, for example, F- , Cl- , Br- , I- Halide such as; OH- ; ClO4- ; FSO3- , ClSO3- , CH3 SO3- , C6 H5 SO3- , CF3 SO3- Such as sulfonic acid ion current; HSO4- , SO42 - Such as sulfate ion current; HCO3- , CO32 - Such as carbonate ion current; H2 PO4- , HPO42- , PO43 - Such as phosphate ion current; PF6- , PF5 OH- Such as fluoro phosphate ion current, [CF3 CF2 )3 PF3 ]- , [CF3 CF2 CF2 )3 PF3 ]- , [(CF3 )2 CF)3 PF3 ]- , [(CF3 )2 CF)2 PF4 ]- , [(CF3 )2 CFCF2 )3 PF3 ]- And [(CF3 )2 CFCF2 )2 PF4 ]- Preparation method of phosphate ion current is at least one selected fluoro; BF4- , B (C6 F5 )4- , B (C6 H4 CF3 )4- Such as borate ion current; AlCl4- ; BiF6 , SbF6- , SbF5 OH- Such as fluoro antimony acid ion current, or AsF6- , AsF5 OH- Such as fluoro arsenic acid ion current flow tides. The present invention but not specifically defined relative anion is used, organic electronic device extend the life time of the foregoing in combination with anionic when, is cured at a low temperature is preferably the structure of the polymerisable disclosure number hereinafter. PF6- , PF5 OH- Such as fluoro phosphate ion current, [CF3 CF2 )3 PF3 ]- , [CF3 CF2 CF2 )3 PF3 ]- , [(CF3 )2 CF)3 PF3 ]- , [(CF3 )2 CF)2 PF4 ]- , [(CF3 )2 CFCF2 )3 PF3 ]- And [(CF3 )2 CFCF2 )2 PF4 ]- Preparation method of phosphate ion current is at least one selected fluoro; BF4- , B (C6 F5 )4- , B (C6 H4 CF3 )4- Such as borate ion current; AlCl4- ; BiF6 , SbF6- , SbF5 OH- Such as fluoro antimony acid ion current Cu2Se. The present invention according to ion compound in, but not specifically defined relative combination of silica relative anion, hole transport compound acts as an electron-accepting compound 1 can be electronic oxidizing, active organic EL element driving voltage, and organic electronic device extend the life time of the foregoing in combination with anionic when, cured at a low temperature as carboxylic step [nyum[nyum] PF disclosure number capable of silica6- , PF5 OH- Such as fluoro phosphate ion current; BF4- , B (C6 F5 ) 4- , B (C6 H4 CF3 )4- Such as borate ion current, SbF6- , SbF5 OH- Such as antimony acid ion current combination of fluoro, will not be [nyum[nyum] with PF6- , PF5 OH- Such as fluoro phosphate ion current; BF4- , B (C6 F5 )4- , B (C6 H4 CF3 )4- Such as borate ion current, SbF6- , SbF5 OH- Preferably such as antimony acid ion current combination of fluoro, carboxylic step [nyum[nyum] B silica (C6 F5 )4- , B (C6 H4 CF3 )4- Such as borate ion current, SbF6- , SbF5 OH- Such as antimony acid ion current combination of fluoro, will not be [nyum[nyum] with B (C6 F5 )4- , B (C6 H4 CF3 )4- Such as borate ion current, SbF6- , SbF5 OH- Such as antimony acid ion current combination of more preferably fluoro. Specifically tree phenyl car [lu[lu] step [nyum[nyum] SbF silica6- Salt, tree phenyl car [lu[lu] step [nyum[nyum] B silica (C6 F5 )4- Salt, N, N - dimethyl will not be [nyum[nyum] with SbF6- Salt, N, N - dimethyl will not be [nyum[nyum] with B (C6 F5 )4- For example salt is cited. Ion compounds, may be used alone and, at a rate of at least one any 2 mixing a retarder. [Charge-transporting compound] In the present invention, charge-transporting compounds, compounds having charge-transporting unit are disclosed. "Charge-transporting unit" RM, hole or electron transporting donating group that has the capability of which, hereinafter, described and detailed against each other. Said charge-transporting unit, and then the hole or electron transporting capability, particularly but not limited to, segments having amine or carbazole, preferably a thiophene, for example, general formula (1a) preferably has a partial structure represented ∼ (7a). [Formula 2] The composition, Ar1 ∼Ar20 Is, independently aryl group or a heteroaryl group of 2 ∼ 30 carbon atoms, or a substituted or nonsubstituted it will be biting, [leyn, it will be biting and [leyn it will raise hetero exhibits. Wherein, the aryl is a stand-alone number 1 hydrogen atom from aromatic hydrocarbons dog a donating group, hetero aryl, hetero atoms in the aromatic compound having a hydrogen atom from a dog donating group number 1 a stand-alone, or R by a goniophotometer. R - R are each independently1 , - OR2 , - SR3 , - OCOR4 , - COOR5 , - SiR6 R7 R8 Or general formula (2a) ∼ (4a) (stage, R1 ∼R8 Is, hydrogen atom, 1 ∼ 22 carbon atoms of linear, cyclic or branched alkyl group, aryl group or heteroaryl group of carbon number 2 ∼ 30 represents) by a goniophotometer. Wherein, a dog and a stand-alone hydrogen atom donating group number 2 it will be biting and [leyn it crawls from aromatic hydrocarbons, heterocyclic it will be biting and [leyn it crawls, an aromatic compound having a hydrogen atom from a donating group number 2 heteroatoms dog stand-alone are disclosed. X is during said R, having one or more hydrogen atoms from group 1, two hydrogen atoms number 1 further exhibits a stand-alone group. In addition, the solubility of the charge-transporting compound in the present invention, film forming polymer or oligomer preferably in terms of the minute particles. In addition, polymer or oligomer preferably including a repeating unit represented by the general formula. [Formula 3] [Formula 4] [Formula 5] [Formula 6] Said formula, Ar1 ∼Ar100 Is, independently aryl group or a heteroaryl group of 2 ∼ 30 carbon atoms, or a substituted or nonsubstituted it will be biting, [leyn, it will be biting and [leyn it will raise hetero exhibits. Wherein, the aryl is a stand-alone number 1 hydrogen atom from aromatic hydrocarbons dog a donating group, hetero aryl, hetero atoms in the aromatic compound having a hydrogen atom from a dog donating group number 1 a stand-alone, or R by a goniophotometer. R - R are each independently1 , - OR2 , - SR3 , - OCOR4 , - COOR5 , - SiR6 R7 R8 Or general formula (2a) ∼ (4a) (stage, R1 ∼R8 Is, hydrogen atom, 1 ∼ 22 carbon atoms of linear, cyclic or branched alkyl group, aryl group or heteroaryl group of carbon number 2 ∼ 30 represents) by a goniophotometer. Wherein, a dog and a stand-alone hydrogen atom donating group number 2 it will be biting and [leyn it crawls from aromatic hydrocarbons, heterocyclic it will be biting and [leyn it crawls, an aromatic compound having a hydrogen atom from a donating group number 2 heteroatoms dog stand-alone are disclosed. X is during said R, having one or more hydrogen atoms from group 1, two hydrogen atoms number 1 further exhibits a stand-alone group. Y is the group represents a substituted 3, Z is 2 exhibits is of substituents. In addition, x is, denotes an integer of 1 or more. In addition, solubility to vary said polymer or oligomer, preferably having at least one 1 "polymerizable substituent". Wherein, said "polymerizable substituent" is, by forming a union between reactor 2 molecules or more molecules which can be substituted group, hereinafter, described and detailed against each other. As said polymerizable substituents, having carbon - carbon multiple bond to (for example, vinyl, acetylene to, vice-reel neel, acrylic, acrylic the [ley sprouting,, acrylic amide, meta [khu[khu] reel, meta the [khu relay sprouting,, meta [khu reel amide, oh [leyn, reel it will know, vinyl ether, vinyl amino group, [phyu reel, to pyrrole, thiophene to, thread roll and the like sound), database (small non-membered ring) having a ring (for example, a cyclopropyl group, cyclo butyl, epoxy, oxetane, [theyn[theyn] diketone, such as epitaxial opinion feed), a lactone, a lactam, or containing a siloxane derivative such as for example is cited. In addition, to the aforementioned outside, an ester bond or an amide bond can be formed on one or combinations of a test. For example, ester with amino group, such as hydroxyl groups with a combination of ester even with each other. As polymerizable substituents, in particular, oxetane, epoxy, vinyl, vinyl ether, acrylic the [ley sprouting,, more preferably the [khu relay sprouting, reactive in terms of metadata, oxetane is preferred in particular. In addition, in the present invention polymerization layer polymer or oligomer, solubility and heat resistance, for regulating electric characteristics, in addition to said repeating units, said it will be biting, [leyn, hetero it will be biting, [leyn, or said copolymer having a general formula of medication is copolymerized as repeating unit even with each other. In this case, copolymer include, random, block or graft copolymer and even, for controlling structure of a light, for example, block-charged even random copolymers are disclosed. In addition, in the present invention polymers for use or oligomer, having a hydroxylated branches, at least one end 3 even with each other. (Solvent) The present invention for use in the solvent, chloroform, methylene chloride, dichloroethane, tetrahydrofuran mixable, toluene, xylene, mesh [thil, not brush, toll-targeting, acetone, methyl ethyl ketone, hops, acetic acid butyl, hops, acetic acid n - butyl, lactic acid ethyl, lactic acid n - butyl, γ butyrolactone, ethyl it will count with brush [pu acetate, phenyl acetic acid, propionic acid phenyl, methyl benzoic acid, benzoic acid ethyl, propyl benzoic acid, benzoic acid n - butyl diphenylmethane, d phenyl ether, N, N - dimethyl formamide, N, N - dimethylacetamide, such as for example ethylene glycol d methyl reel [thu phosphorus is cited. They may be used alone and which 1 species, at least one retarder 2 using any combinations and proportions. (Ratio) Cationic compound is, charge-transporting compound which gives 100 parts by mass, 0. 01 parts by mass to 50 parts by mass is preferably, 0. 05 parts by mass to more preferably 25 parts by mass, 0. 1 parts by mass to 20 parts by mass of is most preferably. Ion compounds ratio 0. 01 parts by mass is obtained without lowering a driving voltage is below, ix. exceeds 50 parts by mass of driving voltage rises. Disclosure number when used as ion compounds, 100 parts by mass of compound having polymerizable substituents, 0. 1 parts by mass to 50 parts by mass of Cu2Se. 0. 1 parts by mass in less than, polymerization is not progress sufficiently, exceeds 50 parts by mass, lower quality number exists in the flow tides. And, ion compounds as in polymerization using the method disclosure number, preferably a solution of a polymerization disclosure. (Other components) Cationic compound is, polymerization disclosure number etc. used as electromagnetic receptor function. These may be used alone and, a plurality combination retarder. In addition, the out-of-polymerization of the present invention disclosure number or electronic receptors retarder. In addition, crosslinked material needed luminescence material a retarder. <Organic electroluminescence element> (Hereinafter, referred to as "organic EL element" by) the organic electroluminescence element of the present invention, number 1 and number 2 of the sun aspects of sun and 2, number 1 from S. first described aspect. [Organic EL element number 1 aspects of] Aspects of organic EL element of the present invention number 1, the aforementioned organic electronic material of the present invention is formed (hereinafter, may be a polymer layer as) characterized as having a. Organic EL element of the present invention, light emitting layer, polymer layer, anode, cathode, without specifically defined then the substrate, hole injection layer, an electron injection layer, hole transporting layer, an electron transport layer has a layer of hydration may comprise other. Hereinafter, detailed each layer are described as follows. [Light emitting layer] Using the light emitting layer is fitted in an opening, and even low molecular weight compounds, polymer or oligomer is even, the center of gravity of dendrimer available disclosed. Fluorescence using include a molecular weight, perylene, coumarin, base [pu, quinacridone, dye laser pigment (for example, with was and it pushed, such as DCM1), aluminum complex [for example, tris (8 - hydroxy hour [khwi[khwi] it teases but the toe) aluminum (III) (Alq3 )], Stilbene, derivatives is cited as an example. Fluorescence using polymer or oligomer as, polyfluorenes, polyphenylene, polyphenylene vinylene (PPV), bladder composition (PVK), fluorene - benzothiadiazole copolymer, fluorene - triphenyl amine copolymer, and their derivatives or mixture can be suitable. On the other hand, recent organic EL for to improve the efficiency, the development of phosphorescent organic EL element also extended by means disclosed. Organic EL phosphor elements, as well as using singlet state energy of the energy of a triplet state possible, increasing it up in accordance with the 100% internal quantum yield principle. Organic EL phosphor elements, including heavy metals such as platinum or iridium metal tightly the system phosphor material emitting phosphor as impurities, against other (non-patent document 3 ∼ 5 reference) host material is a phosphorescent light-emitting by doping. Organic EL element of the present invention even, in terms of a high, preferably phosphorescent material that light emitting layer. As phosphor material, such as metal complexes such as metal including Ir or Pt center can be preferably used. Specifically, Ir complexes include, for example, blue (pic) performing FIr ' iridium (III) bis [4, 6 - difluorophenyl) - pyridazinone - N films, C2 ] Blood collie four [thu ', green luminescent performing Ir (ppy)3 'Fac - tris (2 - phenylpyridine) iridium' (non-patent document 4 reference) or Adachi et al. , Appl. Phys. Lett. , 78 no. 11, 2001, 1622 (btp) performing a red light emission2 Ir (acac) {bis '2 - (2' - benzo [4, 5 - α] thienyl) pyridazinone - N films, C3 ' iridium (acetylacetonate)}, Ir (piq)3 'Tris (1 - phenyl isoquinoline) iridium' such as for example is cited. Pt complexes include, for example, red light emission performing preparation of ethyl 2, 3, 7, 8, 12, 13, 17, 18 - - 21H, 23H - pin [phul mote [nyum etc. formoterol (PtOEP). Phosphor material, molecular weight or dry [tu kind it is burnt (dendride compound), for example, iridium nuclear dendrimer can be used. In addition, their derivatives also can be preferably used. In addition, light emitting layer of the phosphor material if, in addition to the phosphor material, preferably including host material. As host material, and even low molecular weight compounds, and even polymer compound, such as dendrimers can be be used. Include a low molecular weight, for example, CBP (4, 4 '- bis (carbazole - 9 - yl) - biphenyl), mCP (1, 3 - bis (9 - carbazole) benzene), CDBP (4, 4' - bis (carbazole - 9 - yl) - 2, 2' - dimethyl biphenyl) can be use, include a, for example, bladder composition, polyphenylene, polyfluorenes etc. can be, derivatives may be used. The light emitting layer, and may be formed by vapor deposition, coating technique formed by a retarder. In the case of forming by coating technique, organic EL element can be formed at high pressure liquid coolant number, more preferably. Light-emitting layer formed according coating technique order, phosphorescent material, host material needed solution including, for example, ink-jet method, casting process, the dipping law, convex plate printing, is formed on a printing, offset printing, lithography, is formed on a shift offset printing, screen printing, gravure printing mode printing, publicly known method such as spin coating of an intended gas (bodies) can be carried out by applying. The coating method such as described above, typically, at a temperature ranging from -20 ∼ + 300 °C, preferably 10 ∼ 100 °C, in particular preferably in 15 ∼ 50 °C which is capable, in addition said solution as solvents usable, but not one specifically number, for example, chloroform, methylene chloride, dichloroethane, tetrahydrofuran mixable, toluene, xylene, mesh [thil, not brush, acetone, methyl ethyl ketone, hops, acetic acid butyl, ethyl it will count with brush [pu acetate, diphenylmethane, d phenyl ether, tetralin etc.. In addition, the after-application, hot plate or oven temperature in the range of + 30 ∼ + 300 °C by number are also comprehensively solvent by heating. [Polymerization layer] The polymerizable layer, organic layer film of the present invention the aforementioned organic electronic material be applied to a substrate are disclosed. And, in the present invention, said organic electronic include a charge-transporting materials, one or more polymerizable substituents 1 preferably having charge-transporting compound, here the charge-transporting compound relative to the layer of polymer including organic electronic material formed using a detailed as follows. Polymer layer RM, specifically, one or more polymerizable substituents 1 having charge-transporting compound including organic electronic material, described carry thin film forming method desired by the coating technique is applied on a base, by light irradiation or heat treatment, charge-transporting compound is a tertiary substituents to reaction to proceed polymerization of, overlaying layer layer (cured) to change solubility are disclosed. As aforementioned, charge-transporting compound is a tertiary substituents to reaction to proceed polymerization of, overlaying layer by change solubility (curing), said layer thermal stability can be improved. In addition, polymerization by lowering solubility, even in the case of forming a light emitting layer coating liquid and polymerized by means of hydration layers not layer is dissolved, said other layer is applied at an exterior of the can. I.e., hereinafter for a multilayer structure can be formed by coating technique, high efficiency, long life organic EL element, high pressure liquid coolant number can be low cost. Polymer layer using charge-transporting compound is, in view of the uniformity of the film, preferably including a repeating unit with hole transport polymer or oligomer. said polymerizable layer, organic EL for, hole injection layer, hole transporting layer, an electron transport layer, and an electron injection layer, luminous efficiency, life characteristics in terms of, in particular hole injection layer, hole transport layers are preferred. Either hole injection layer and hole transport layer and a polymeric layer 117, both polymer layer 117 with each other. In addition, film thickness of these layers, particularly number is not available but a smooth, preferably in 10 ∼ 100 nm, more preferably in 20 ∼ 60 nm, most preferably one having 20 ∼ 40 nm. In addition, said polymerizable layer, more preferably stacked adjacent phosphor material including light emitting layer. the polymerizable layer, a light-emitting phosphor material, impact small degradation, element is emitting efficiency can be improved service life are disclosed. [Substrate] Organic EL element of the present invention can be used substrate as, glass, plastic particularly as a search without limit, in addition, transparent but not specially number if a smooth, glass, quartz, light transmitting resin films are preferably used. When using the resin film, organic EL element (flexibility) can be flexible-imparting, preferably in particular. As the resin film, for example, polyethylene terephthalate (PET), polyethylene naphthalate (PEN), polyethersulfone (PES), polyetherimide, polyether ether ketone, polyphenylene snow feed, polyarylate, polyimide, polycarbonate (PC), cellulose tree acetate (TAC), cellulose acetate pro blooms four the [thu consists of film etc. (CAP). In addition, as the resin film, such as the permeation of water vapor or oxygen for number billion, inorganic material such as silicon oxide or silicon nitride coating resin film to the use of a retarder. [Cathode] As cathode material, for example, Li, Ca, Mg, Al, In, Cs, Ba, Mg/Ag, LiF, preferably a metal or metal alloy such as CsF. [Anode] As the anode, metal (e.g., Au) or metal conductivity which have different material, for example, oxide (for example, ITO: indium oxide/tin oxide), conductive polymer (e.g., polythiophene (PEDOT: PSS) - polystyrene the mote [leyn[leyn] the opinion phone it buys mixture can be using. [Electron transport layer, electron injection layer] An electron transport layer, electron injection layer uses, for example, phenanthroline derivatives (for example, 2, 9 - dimethyl - 4, 7 - diphenyl - 1, 10 - phenanthroline (BCP), bipyridine derivatives, nitro substituted fluorene derivatives, d phenyl [khwi rice field derivatives, thio blood column d jade hour [tu derivatives, perylene naphthalene carboxylic acid anhydride such as heterocyclic 2, carbodiimide, the flue five [ley neel the methane which is burnt derivatives, derivatives do not sprout the [khwi roh d methane and inside [thu, oxadiazole derivatives [2 - (4 - biphenyl) - 5 - (4 a-tert - butyl phenyl - l-a 1, 3, 4 - oxadiazole] (PBD), aluminum complex [for example, tris (8 - hydroxy) aluminum (III) (Alq3 )] Etc.. In addition, in said oxadiazole derivatives, oxadiazole ring oxygen atoms sulfur substitutionally replaced motors in diazol derivatives, that are known to be used quinoxaline derivatives having electron as the [khwi stipend the annularity which it saves can be. [Organic EL element number 2 aspects of] Aspects of organic EL element of the present invention number 2, is composed of a multilayer structure, adjacent 2 dog layer of said multilayer structure, the hole-transporting compound including each substituent polymerizable mixture is formed by coating technique, each hole-transporting compound by polymerizing etc. and the heat dissipating body. Hereinafter, solar as well as number 1, the hole-transporting compound including substituent polymerizable mixture is formed by coating technique and, said hole-transporting compound of polymeric solubility polymerizing layer changing layer as the substrate. Aspects of organic electro-light-emitting device of the present invention number 2 multilayer structure but, respective ones of said multilayer structure are various layer, light emitting layer, polymer layer, anode, cathode, without specifically defined then the substrate, hole injection layer, an electron injection layer, hole transporting layer, an electron transport layer, a hole blocking layer, electron blocking layer, stop layer having a layer of hydration in exciton ([...]) may comprise other. Hereinafter, detailed each layer are described as follows. [Polymerization layer] Polymer layer, i.e. substituent having hole transporting compound layer is formed by using a mixture including, specifically, the hole-transporting compound including substituent polymerizable mixture, desired by a coating technique is applied on a base, by light irradiation or heat treatment, the reaction to proceed polymerization of hole transporting compound a tertiary substituents, overlaying layer layer (cured) to change solubility are disclosed. As aforementioned, the hole-transporting compound is a tertiary substituents polymerization of reaction to proceed, overlaying layer by change solubility (curing), said layer thermal stability can be improved. In addition, polymerization by lowering solubility, even in the case of forming a light emitting layer further polymerized by means of hydration layers since the coating liquid and do not dissolve layer, said other layer is applied at an exterior of the can. I.e., hereinafter for a multilayer structure can be formed by coating technique, high efficiency, long life organic EL element, high pressure liquid coolant number can be low cost. The dielectric layer polymer comprise a hole-transporting compound is, in view of the uniformity of the film, preferably including a repeating unit with hole transport polymer or oligomer. In addition, at least one polymerizable substituent the hole-transporting compound 2 any defoamer and even when used, the hole-transporting compound is mixed with the polymerizable substituent not used a retarder. said polymerizable layer, organic EL for, hole injection layer, hole transporting layer, an electron transport layer, and an electron injection layer, luminous efficiency, life characteristics in terms of, in particular hole injection layer, hole transport layers are preferred. Either hole injection layer and hole transport layer and a polymeric layer 117, both polymer layer 117 with each other. In addition, film thickness of these layers, particularly number is not available but a smooth, preferably in 10 ∼ 100 nm, more preferably in 20 ∼ 60 nm, most preferably one having 20 ∼ 40 nm. The present invention according to the "adjacent 2 wustite", hole injection layer layer, hole transport layer preferably is either. In addition, polymerization or polymerization disclosure number disclosure number generated by decomposition of acid or polymerizing disclosure number are, organic EL element layer compounds, in particular the light command or the reaction mixture may be a disclosed. Therefore, during wustite adjacent 2, anode disclosure number near mixing only polymer layer, or other layer by layer by polymerizing polymer diffusion from active ingredient disclosure number, while also being capable to form a multilayer structure, the influence of polymer light-emitting layer is less disclosure number, organic EL element number can be luminous efficiency or life high pressure liquid coolant. In the present invention using the hole-transporting compound polymerizable substituent including mixture is, according to the number of fluorine-containing polymer layer applied properties needed, leveling number (leveling agent) number or vesicles ([...]), crosslinked number, an electron-accepting compound, hole transporting donating group of high molecular weight polymer that has the capability of including a retarder. In the present invention polymerizable substituent using the hole-transporting compound of "polymerizable substituent" implies, the reactor can be substituted by 2 molecules or more molecules forming a union between a paper-making, hereinafter, detailed as follows. As said polymerizable substituents, "polymerizable substituent" of the aforementioned organic electronic material of the present invention and description of the same, also in preferred embodiments are the same. Polymerizable substituents, coupled between 2 molecules or more molecules formed of polymers prepared by coating, the coating film solubility change (curing) could be bonded each other. Paint film to vary the solubility, 1 polymerizable substituents preferably being at least 2 per molecule. In the present invention using the "hole-transporting compound", that has the capability of transporting hole donating group including compounds which, hereinafter, detailed as follows. the "hole transporting donating group that has the capability of", and then the hole transporting capability, but not one specifically number, aryl amine, carbazole, preferably a thiophene, for example, by the general formula (1) preferably has a partial structure represented ∼ (58). [Formula 7] [Formula 8] [Formula 9] [Formula 10] [Formula 11] The composition, E are each independently - R1 , - OR2 , - SR3 , - OCOR4 , - COOR5 , - SiR6 R7 R8 Or the general formula (59) (61) ∼ (stage, R1 ∼R11 Is, hydrogen atom, 1 ∼ 22 carbon atoms of linear, cyclic or branched alkyl group, aryl group or heteroaryl group of carbon number 2 ∼ 30 represents, a and b and c is, denotes an integer of 1 or more. Wherein, the aryl, a stand-alone number 1 hydrogen atom from aromatic hydrocarbons donating group and a dog, and substituent group, hetero aryl, hetero atoms in the aromatic compound having a hydrogen atom from a dog number 1 is a stand-alone donating group, substituent group other), or "polymerizable substituent" exhibits either. The Ar, 2 ∼ 30 carbon atoms independently of it will be biting, [leyn, it will be biting and [leyn it will raise or hetero-aryl exhibits. It will be biting and [leyn it crawls from aromatic hydrocarbons and hydrogen atom donating group substituent may comprise a dog number 2 is a stand-alone, for example, phenylene, it will be a non-phenyl d, it will be a phenyl d, naphthalene diyl-, anthracene diyl-, 2 it will be a d it counts, the flue they will be five [leyn d, etc. lung difficulty [thu it will be a d. Hetero aryl, hetero atoms in the aromatic compound having a hydrogen atom from a donating group substituent may comprise a stand-alone and dog number 2 which, for example, pyridine diyl-, it will be an aphid antagonist activity, quinoline diyl-, ISO it will be a quinoline d, arc [tin it will be a d, phenanthroline diyl-, furan diyl-, pyrrole diyl-, may be thio snoop, oxa it will be a D it will doze, oxadiazole diyl-, will be and oh it will be a d it will doze thiazole, triazole it will be a D it will doze, benzo the jade company it will be a d it will doze, benzo jade company d oh it will be a d it will doze, benzo mote ada oh it will be a d it will doze, benzo tree oh it will be a d it will doze, benzo it will be a mote five pen d etc.. X and Z is, independently of said R, one or more hydrogen atoms from groups having 1, further number of hydrogen atoms 1 represents a stand-alone group, X denotes an integer of 0 ∼ 2 is. During said X or Y is Z, 2 hydrogen atoms from hydrogen atoms having at least one of a stand-alone exhibits number into 2 groups. [Formula 12] The "hole-transporting compound", more preferably film uniformity in terms of oligomer or polymer. The number average molecular weight of said oligomer or polymer, preferably 1,000 or more in 1,000,000 hereinafter, 1,000 or more, more preferably 500,000 hereinafter in, 2,000 or more, most preferably one having 200,000 hereinafter. A number average molecular weight is below 1,000 number stable amorphous silicon film crystallized easily tends, lowers the workability in application number exceeds 1,000,000 for solubility for minute superfine substrate. Said polymer or oligomer is contained in general formula (1a) preferably including a repeating unit represented by ∼ (84a). And, general formula (1a) (84a) of ∼, Ar, E, X, x is said general formula (1) (60) ∼ of Ar, E, X, x meanings the same are disclosed. [Formula 13] [Formula 14] [Formula 15] [Formula 16] [Formula 17] [Formula 18] [Formula 19] [Formula 20] [Formula 21] [Formula 22] [Formula 23] [Formula 24] In addition, said polymer or oligomer, solubility and heat resistance, for regulating electric characteristics, in addition to said repeating units, said it will be biting, [leyn, hetero it will be biting, [leyn, or a group represented by the general formula (C1) (C30) ∼ copolymer having copolymerized as repeating unit structure even with each other. In this case, copolymer include, random, block or graft copolymer and even, for controlling structure of a light, for example, block-charged even random copolymers are disclosed. In addition, in the present invention polymers for use or oligomer, having a hydroxylated branches, at least one end 3 may have other. [Formula 25] The present invention according to "the hole-transporting compound mixture including polymerizable substituent" is, polymerization can be combined further disclosure number may be filled. As a polymeric disclosure number, heat, light, microwave, radiation, such as by the application of electron beam, if the expression of polymerizing polymerizable substituents ability and, particularly but not one number, so that the polymerization by heating or light irradiation and/disclosure preferably, more preferably to polymerization by heat disclosure. Disclosure number as said polymerization, polymerization by light irradiation and/or heating as to disclosure, disclosure is provided in addition to polymerization by heat, preferably five [nyum salts. Wherein, in the present invention RM onium salt, for example, sulfonium, five degree [nyum[nyum], carboxylic hemp cloth [nyum, selenium, ammonium, phosphonium, bis nothing small [nyum for which the relative anion such as silica joined substrate. As the relative anion, the aforementioned organic electronic material of the present invention description of relative ion compound of anions are the same. Sulfonium ions are preferably, phenyl opinion gun [nyum tree, tree - p - [thol[thol] Reel opinion gun [nyum[nyum], tree - o - [thol[thol] Reel opinion gun [nyum[nyum], tris (4 - methoxyphenyl) sulfonium, me [phu d phenyl opinion gun [nyum 1 -, 2 - me [phu d phenyl opinion gun [nyum, tris (4 - fluorophenyl) sulfonium, tree - 1 - me [phu opinion gun [nyum, tree - 2 - me [phu opinion gun [nyum, tris (4 - hydroxyphenyl) sulfonium, 4 - (phenylthio) phenyl d phenyl opinion gun [nyum[nyum], 4 - di - p - [thol[thol] Reel opinion gun [nyum[nyum] (p - [thol reel mote five) phenyl, 4 - (4 - maul [thok hour phenyl mote five) phenyl bis (4 - methoxyphenyl) sulfonium, 4 - (phenylthio) phenyl bis (4 - fluorophenyl) sulfonium, 4 - (phenylthio) phenyl bis (4 - methoxyphenyl) sulfonium, 4 - (phenylthio) phenyl di - p - [thol[thol] Reel opinion gun [nyum[nyum], bis [4 - (d phenyl opinion gun [ni five) phenyl] opinion blood set, bis '4 - {bis [4 - (2 - hydroxy in at the time of [thok[thok] at the time of) phenyl] sulfonyl [ni[ni] five} phenyl' opinion blood set, bis {4 - [bis (4 - fluorophenyl) sulfonyl [ni[ni] five] phenyl} opinion blood set, bis {4 - [bis (4 - methylphenyl) sulfonyl [ni[ni] five] phenyl} opinion blood for use in, bis {4 - [bis (4 - methoxyphenyl) sulfonyl [ni[ni] five] phenyl} opinion blood set, 4 - (4 - benzoyl - 2 - 3.5 chloro) phenyl bis (4 - fluorophenyl) sulfonium, 4 - (4 - benzoyl - 2 - 3.5 chloro) phenyl d phenyl opinion gun [nyum[nyum], 4 - (4 - benzoyl 3.5) phenyl bis (4 - fluorophenyl) sulfonium, 4 - (4 - benzoyl 3.5) phenyl d phenyl opinion gun [nyum[nyum], d high draw anthracene - 9, 10 - oxo - 10 - 7 - thia - 9 - isopropyl - 2 - one di - p - [thol[thol] Reel opinion gun [nyum[nyum], d high draw anthracene - 9 - thia - 9, 10 - 7 - isopropyl - 2 - oxo - 10 - one d phenyl sulfonium, the mote jade it buys ton 2 - [(di - p - tolyl) sulfonyl [ni[ni] five], 2 - [(diphenyl) sulfonyl [ni[ni] five] the mote jade it buys ton, 4 - [4 - (4 a-tert - butyl benzoyl) phenylthio] phenyl di - p - [thol[thol] Reel opinion gun [nyum[nyum], 4 - [4 - (4 a-tert - butyl benzoyl) phenylthio] phenyl d phenyl opinion gun [nyum[nyum], 4 - [4 - (benzoyl 3.5)] phenyl di - p - [thol[thol] Reel opinion gun [nyum[nyum], 4 - [4 - (benzoyl 3.5)] phenyl d phenyl opinion gun [nyum[nyum], 5 - (4 - methoxyphenyl) [thu[thu] rhenium inside mote, 5 - phenyl [thu[thu] rhenium inside mote, 5 - [thol [thu rhenium inside reel mote, 5 - (4 - [thok[thok] Hour phenyl) [thu[thu] rhenium inside mote, 5 - (2, 4, 6 - trimethylphenyl) such as triazole reel opinion gun [nyum[nyum][thu[thu] rhenium inside mote; the d phenyl lung the opinion gun which will be born [nyum, diphenyl 4 - knit with the lung the opinion gun which will be born [nyum, the d phenyl it cuts the qualitative opinion gun [nyum, such as reel opinion gun [nyum[nyum] process to d phenyl methyl opinion gun [nyum; the methyl it cuts the qualitative opinion gun [nyum phenyl, 4 - hydroxy hour phenyl methyl it cuts the qualitative opinion gun [nyum, the maul [thok hour phenyl methyl it cuts the qualitative opinion gun [nyum 4 -, 4 - the carbonyl jade hour phenyl methyl it cuts the qualitative opinion gun [nyum acetamide, 2 - me [phu the methyl it cuts the qualitative opinion gun [nyum, FR 2 - (1 - ethoxycarbonyl) ethyl opinion gun [nyum, the methyl lung the opinion gun which will be born [nyum phenyl, 4 - hydroxy hour phenyl methyl lung the opinion gun which will be born [nyum, the maul [thok hour phenyl methyl lung the opinion gun which will be born [nyum 4 -, 4 - the carbonyl jade hour phenyl methyl lung the opinion gun which will be born [nyum acetamide, 2 - me [phu the methyl lung the opinion gun which will be born [nyum, it will be burnt the lung the opinion gun which will be born [nyum 2 - me [phu jade other, do not sprout three neel methyl lungs the opinion gun which will be born [nyum 9 - such as mono aryl sulfonium; the lung the opinion gun which will be born [nyum dimethyl, lung or thread tetra high draw mote five lung [nyum, it cuts the qualitative opinion gun [nyum dimethyl, tetra high draw mote five lung [nyum benzyl, such as such as for example alkyl opinion gun [nyum include the preparation of the methyl lung which will be burnt the opinion gun which will be born [nyum tree, they disclosed hereinafter of the nucleotide. In respect of the reel opinion gun [nyum triazole, U.S. patent number 4231951 call specification, U.S. patent number 4256828 call specification, Japanese patent application publication number plain 7 - 61964 call Official Gazette, Japanese patent application publication number plain 8 - 165290 call Official Gazette, Japanese patent application publication number plain 7 - 10914 call Official Gazette, Japanese patent application publication number plain 7 - 25922 call Official Gazette, Japanese patent application publication number plain 8 - 27208 call Official Gazette, Japanese patent application publication number plain 8 - 27209 call Official Gazette, Japanese patent application publication number plain 8 - 165290 call Official Gazette, Japanese patent application publication number plain 8 - 301991 call Official Gazette, Japanese patent application publication number plain 9 - 143212 call Official Gazette, Japanese patent application publication number plain 9 - 278813 call Official Gazette, Japanese patent application publication number plain 10 - 7680 call Official Gazette, Japanese patent application publication number plain 10 - 287643 call Official Gazette, Japanese patent application publication number plain 10 - 245378 call Official Gazette, Japanese patent application publication number plain 8 - 157510 call Official Gazette, Japanese patent application publication number plain 10 - 204083 call Official Gazette, Japanese patent application publication number plain 8 - 245566 call Official Gazette, Japanese patent application publication number plain 8 - 157451 call Official Gazette, Japanese patent application publication number plain 7 - 324069 call Official Gazette, Japanese patent application publication number plain 9 - 268205 call Official Gazette, Japanese patent application publication number plain 9 - 278935 call Official Gazette, Japanese patent application publication number 2001 - 288205 call Official Gazette, Japanese patent application publication number plain 11 - 80118 call Official Gazette, Japanese patent application publication number plain 10 - 182825 call Official Gazette, Japanese patent application publication number plain 10 - 330353 call Official Gazette, Japanese patent application publication number plain 10 - 152495 call Official Gazette, Japanese patent application publication number plain 5 - 239213 call Official Gazette, Japanese patent application publication number plain 7 - 333834 call Official Gazette, Japanese patent application publication number plain 9 - 12537 call Official Gazette, Japanese patent application publication number plain 8 - 325259 call Official Gazette, Japanese patent application publication number plain 8 - 160606 call Official Gazette, Official Gazette such as Japanese patent application publication number 2000 - 186071 call (call specification U.S. patent number 6368769); for the diaryl reel opinion gun [nyum, Japanese patent application publication number plain 7 - 300504 call Official Gazette, Japanese patent application publication number 64 - 45357 bovine call Official Gazette, Japanese patent application publication number 64 - 29419 call such as bovine Official Gazette; mono biting opinion gun [nyum respect, Japanese patent application publication number plain 6 - 345726 call Official Gazette, Japanese patent application publication number plain 8 - 325225 call Official Gazette, Japanese patent application publication number call Official Gazette (U.S. patent number 6093753 call specification) 9 - 118663 plain, Japanese patent application publication number plain 2 - 196812 call Official Gazette, Japanese patent application publication number plain 2 - 1470 call Official Gazette, Japanese patent application publication number plain 2 - 196812 call Official Gazette, Japanese patent application publication number plain 3 - 237107 call Official Gazette, Japanese patent application publication number plain 3 - 17101 call Official Gazette, Japanese patent application publication number plain 6 - 228086 call Official Gazette, Japanese patent application publication number plain 10 - 152469 call Official Gazette, Japanese patent application publication number plain 7 - 300505 call Official Gazette, Japanese patent application publication number 2003 - 277353 call Official Gazette, like Japanese patent application publication number 2003 - 277352 call Official Gazette; alkyl opinion gun [nyum tree respect, Japanese patent application publication number plain 4 - 308563 call Official Gazette, Japanese patent application publication number plain 5 - 140210 call Official Gazette, Japanese patent application publication number plain 5 - 140209 call Official Gazette, Japanese patent application publication number plain 5 - 230189 call Official Gazette, Japanese patent application publication number plain 6 - 271532 call Official Gazette, Japanese patent application publication number 58 - 37003 bovine call Official Gazette, Japanese patent application publication number plain 2 - 178303 call Official Gazette, Japanese patent application publication number plain 10 - 338688 call Official Gazette, Japanese patent application publication number plain 9 - 328506 call Official Gazette, Japanese patent application publication number plain 11 - 228534 call Official Gazette, Japanese patent application publication number plain 8 - 27102 call Official Gazette, Japanese patent application publication number plain 7 - 333834 call Official Gazette, Japanese patent application publication number plain 5 - 222167 call Official Gazette, Japanese patent application publication number plain 11 - 21307 call Official Gazette, Japanese patent application publication number call Official Gazette 11 - 35613 plain, for example such as U.S. patent number 6031014 call specification is cited. five degree [nyum[nyum] ions are preferably, five degree d phenyl [nyum, di - p - [thol[thol] Five degree reel [nyum[nyum], bis (4 - accumulation of thread phenyl) five degree [nyum[nyum], bis (4 - methoxyphenyl) five degree [nyum[nyum], (4 - jade [thil jade hour phenyl) phenyl five degree [nyum[nyum], bis (4 - it will be burnt jade hour phenyl) five degree [nyum[nyum], 4 - (2 - hydroxy hour tetra the jade city which will be burnt) five degree phenyl [nyum phenyl, 4 - isopropyl phenyl (p - tolyl) five degree [nyum[nyum], such as isobutyl phenyl (p - tolyl) and five degree [nyum[nyum] in size, they, Macromolecules, 10, 1307 (1977), Japanese patent application publication number plain 6 - 184170 call Official Gazette, U.S. patent number 4256828 call specification, U.S. patent number 4351708 call specification, Japanese patent application publication number 56 - 135519 bovine call Official Gazette, Japanese patent application publication number 58 - 38350 bovine call Official Gazette, Japanese patent application publication number plain 10 - 195117 call Official Gazette, Japanese patent application publication number 2001 - 139539 call Official Gazette, Japanese patent application publication number 2000 - 510516 call Official Gazette, like Japanese patent application publication number 2000 - 119306 call Official Gazette disclosed. Carboxylic cation as the hemp cloth [nyum, tree phenyl car [lu hemp cloth [nyum cation, tree (methylphenyl) carboxylic hemp cloth [nyum cation, tree (dimethylphenyl) cation such as hemp cloth [nyum carboxylic acid is cited example reel car [lu hemp cloth [nyum triazole. Selenium ions are preferably, triphenyl selenium, tree - p - [thol the reel the rhenium which it will count, tree - o - the [thu the reel the rhenium which it will count, tris (4 - methoxyphenyl) selenium, 1 - me [phu the d phenyl the rhenium which it will count, tris (4 - fluorophenyl) selenium, tree - 1 - me [phu the rhenium which it will count, tree - 2 - me [phu the rhenium which it will count, tris (4 - hydroxyphenyl) selenium, the d phenyl the rhenium which it will count 4 - (phenylthio) phenyl, 4 - (p - [thol reel mote five) phenyl di - p - [thol the reel the rhenium which it will count the reel the rhenium which it will count such as triazole; the d phenyl lung will be born and the rhenium which it will count, the d phenyl it cuts quality the rhenium which it will count, such as the reel the rhenium which it will count process to the d phenyl methyl the rhenium which it will count; selenium phenylmethyl benzyl, 4 - hydroxyphenyl methylbenoyl selenium, phenylmethyl lung or thread selenium, selenium lung or thread methyl 4 - hydroxyphenyl, 4 - mono will be biting and the rhenium which it will count of the maul [thok hour phenyl methyl lung will be born and the rhenium which it will count; dimethyl lung or thread selenium, the tetra high draw the leno lung which it will count [nyum lung or thread, dimethyl [...] it cuts quality it will count, the tetra high draw the leno lung which it will count [nyum benzyl, alkyl for example methyl lung or thread include the preparation of it will be burnt and selenium and selenium, they Japanese patent application publication number 50 - 151997 bovine call Official Gazette, Japanese patent application publication number 50 - 151976 bovine Official Gazette call, Japanese patent application publication number 53 - 22597 call like bovine Official Gazette disclosed. Ammonium ions are preferably, for example, tetramethyl ammonium, ethyl tree methyl ammonium, d ethyl D methyl ammonium, tri-ethyl methyl ammonium, tetra ethyl ammonium, trimethyl - n - propyl ammonium, trimethyl isopropyl ammonium, trimethyl - n - butyl ammonium, trimethyl isobutyl ammonium, trimethyl - tert - butyl ammonium, trimethyl - n - [heyk[heyk] Thread ammonium, dimethyl di - n - propyl ammonium, dimethyl dee small pro the ammonium which will bloom, dimethyl - n - propyl isopropyl ammonium, methyl tree - n - propyl ammonium, tetraalkylammonium such as methyl tree cow pro the ammonium which will bloom; N, N - dimethyl pyrrole lithium, N - ethyl - N - methyl pyrrolidone lithium, N, N - d ethyl blood raleigh [tyum pyrrole such as lithium; N, N'- dimethyl already will be and the loom which will doze, N, N' - the d ethyl already will be and the loom which will doze, N - ethyl - N '- methyl already will be and the loom which will doze, 1, 2, 3 - the methyl already will be and the loom which will doze tree, tree the methyl already will be and the loom which will doze 1, 3, 4 -, 1, 3 - diethyl - 2 - methyl already will be and the loom which will doze, 1, 3 - diethyl - 4 - methyl already will be and the loom which will doze, 1, 2, 3, 4 - tetra the methyl already will be and the loom which will doze such as imidazolium; N, N' - dimethyl tetra high draw blood midi [nyum, N, N'- d ethyl tetra high draw blood midi [nyum, N - ethyl - N' - methyl tetra high draw blood midi [nyum, 1, 2, 3 - tree such as tetra methyl tetra high draw blood midi [nyum high draw blood midi [nyum[nyum]; N, N'- dimethyl wool [lu pulley [nyum, N - ethyl - N - methyl wool [lu pulley [nyum, N, d ethyl wool [lu pulley [nyum N - know of pulley [nyum; N, N - dimethyl blood ferri D [nyum[nyum], N - ethyl - N' - methyl blood ferri D [nyum[nyum], N, such as N'- d ethyl blood ferri d [nyum d [nyum piperidinyl; blood [tyum[tyum] N - methyl, ethyl blood [tyum[tyum] N -, N a-n - propyl blood [tyum[tyum], pro blood it will bloom [tyum N - isocyanate, N a-n - butyl blood [tyum[tyum], blood [tyum[tyum] N - benzyl, pyridyl [...]lung or threadblood [tyum[tyum] N - such as; N, N' - dimethyl already will be and the loom which will doze, N - ethyl - N - methyl already will be and the loom which will doze, N, N' - the d ethyl already will be and the loom which will doze, 1, 2 - diethyl - 3 - methyl already will be and the loom which will doze, 1, 3 - diethyl - 2 - methyl already will be and the loom which will doze, 1 - methyl - 3 a-n - propyl - 2, 4 - dimethyl already will be and the loom which will doze such as imidazolium; [khwi[khwi] glow loom N - methyl, ethyl [khwi[khwi] glow loom N -, N a-n - propyl [khwi[khwi] glow loom, N - ISO pro the [khwi glow loom which will bloom, [khwi[khwi] glow loom N a-n - butyl, benzyl N - [khwi[khwi] glow loom, such as N - lung or thread[khwi[khwi] glow loom[khwi[khwi] glow loom; this cow [khwi[khwi] glow loom N - methyl, ethyl this cow [khwi[khwi] glow loom N -, N a-n - propyl this cow [khwi[khwi] glow loom, N - ISO pro this cow [khwi glow loom which will bloom, this cow [khwi[khwi] glow loom N a-n - butyl, benzyl N - this cow [khwi[khwi] glow loom, such as isoforms N - lung or threadthis cow [khwi[khwi] glow loom[khwi[khwi] glow loom; it cuts trillion motes oh trillion [nyum benzyl, such as it cuts trillion motes oh trillion [nyum lung or thread trillion [nyum thiazole; benzyl arc lithium, lung or thread arc lithium such as arc lithium pin is. They, U.S. patent number 4069055 call specification, Japanese patent number 2519480 call Official Gazette, Japanese patent application publication number plain 5 - 222112 call Official Gazette, Japanese patent application publication number plain 5 - 222111 call Official Gazette, Japanese patent application publication number plain 5 - 262813 call Official Gazette, Japanese patent application publication number plain 5 - 255256 call Official Gazette, Japanese patent application publication number plain 7 - 109303 call Official Gazette, Japanese patent application publication number plain 10 - 101718 call Official Gazette, Japanese patent application publication number plain 2 - 268173 call Official Gazette, Japanese patent application publication number plain 9 - 328507 call Official Gazette, Japanese patent application publication number plain 5 - 132461 call Official Gazette, Japanese patent application publication number plain 9 - 221652 call Official Gazette, Japanese patent application publication number plain 7 - 43854 call Official Gazette, Japanese patent application publication number plain 7 - 43901 call Official Gazette, Japanese patent application publication number plain 5 - 262813 call Official Gazette, Japanese patent application publication number plain 4 - 327574 call Official Gazette, Japanese patent application publication number plain 2 - 43202 call Official Gazette, Japanese patent application publication number 60 - 203628 bovine call Official Gazette, Japanese patent application publication number 57 - 209931 bovine call Official Gazette, disclosed Japanese patent application publication number 9 - 221652 like plain call Official Gazette. Phosphonium ions are preferably, for example, tetra phenyl gun [su gun [nyum, 2 - p - [thol[thol] Reel gun [su[su] gun [nyum[nyum], tetrakis (2 - methoxyphenyl) phosphonium, tetrakis (3 - methoxyphenyl) phosphonium, tetrakis (4 - methoxyphenyl) phosphonium such as tetra biting gun [su gun [nyum; the phenyl it cuts the qualitative gun [su gun [nyum tree, tree the phenyl lung the gun [su gun which will be born [nyum, phenyl methyl gun [su gun [nyum tree, tree such as triazole reel gun [su gun [nyum phenyl butyl gun [su gun [nyum; [thil[thil] It cuts the qualitative gun [su[su] gun [nyum[nyum] tree, tree the butyl it cuts the qualitative gun [su gun [nyum, tetra ethyl gun [su gun [nyum, tetra butyl gun [su[su] gun [nyum[nyum], tetra [heyk[heyk] Thread gun [su[su] gun [nyum[nyum], tree [thil[thil] The lung the gun [su[su] gun which will be born [nyum[nyum], tree such as the butyl lung the gun [su gun which will be born [nyum tetraalkylphosphonium etc. 2. They, Japanese patent application publication number plain 6 - 157624 call Official Gazette, Japanese patent application publication number plain 5 - 105692 call Official Gazette, Japanese patent application publication number plain 7 - 82283 call Official Gazette, Japanese patent application publication number plain 9 - 202873 like call Official Gazette disclosed. Bis nothing small [nyum ions are preferably, for example, disclosed in Japanese patent application publication number 2008 - 214330 call Official Gazette. Using paclitaxel act as in the present invention, the aforementioned relative cation and mating with the anion combustion chamber. The combination of charge balancing number combinations which can specifically if not one but, electronic oxidizing 1 hole transporting compound, organic EL element active driving voltage, PF6- , PF5 OH- Such as fluoro phosphate ion current, [(CF3 CF2 )3 PF3 ]- , [(CF3 CF2 CF2 )3 PF3 ]- , [( (CF3 )2 CF)3 PF3 ]- , [( (CF3 )2 CF)2 PF4 ]- , [( (CF3 )2 CFCF2 )3 PF3 ]- And [( (CF3 )2 CFCF2 )2 PF4 ]- Preparation method of phosphate ion current is at least one selected fluoro; BF4- , B (C6 F5 )4- , B (C6 H4 CF3 )4- Such as borate ion current, SbF6- , SbF5 OH- Preferably such as fluoro antimony acid ion for increasing, in addition polymerizable substituent charge-transporting compound thin film of high pressure liquid coolant at a low temperature for number, sulfonium, five degree [nyum[nyum], hemp cloth [nyum carboxylic acid, more preferably the combination of cation such as ammonium, organic EL element in order to life of hemp cloth [nyum carboxylic acid, ammonium silica B (C6 F5 )4- , B (C6 H4 CF3 )4- Such as borate ion current, SbF6- , SbF5 OH- Such as fluoro antimony acid ion current, F6- , PF5 OH- Such as fluoro phosphate ion current, [(CF3 CF2 )3 PF3 ]- , [(CF3 CF2 CF2 )3 PF3 ]- , [( (CF3 )2 CF)3 PF3 ]- , [( (CF3 )2 CF)2 PF4 ]- , [( (CF3 )2 CFCF2 )3 PF3 ]- And [( (CF3 )2 CFCF2 )2 PF4 ]- Preparation method of phosphate ion current combination of at least one selected from fluoro more preferably disclosed. In particular, polymerization as disclosure number, onium salt among other things, carbon cation, cationic nitrogen, oxygen cation, and selected from the group consisting of transition metal cations is used cations having having ion compound, i.e. the aforementioned of the present invention description of the preferred ion compound of organic electronic material. (Solvent) Polymer layer coating liquid for the solvent crude number, number but not particularly smooth, specifically, methanol, ethanol, isopropyl alcohol with at least one alcohol, pentane, hexane, octane of alkanes, cyclic alkanes such as cyclohexane, benzene, toluene, xylene, mesh [thil, tetralin, diphenylmethane of aromatic solvents, ethylene glycol dimethyl ether, ethylene glycol d ethyl ether, propylene glycol monomethyl ether acetate such as aliphaticether - 1 -, 1, 2 - d maul [thok[thok] hour benzene, 1, 3 - d maul [thok[thok] hour benzene, not brush, toll-targeting, 2 - maul [thok[thok] city [thol[thol] base n, n maul [thok[thok] city [thol[thol] base 3 -, 4 - maul [thok[thok] city [thol[thol] base n, 2, 3 - dimethyl it knows the brush, 2, 4 - dimethyl it knows the brush such as aromatic ether, hops, acetic acid n - butyl, lactic acid ethyl, an aliphatic ester of lactic acid n - butyl, phenyl acetic acid, propionic acid phenyl, benzoic acid methyl, ethyl benzoic acid, benzoic acid propyl, n - butyl benzoic acid such as aromatic ester, N, N - dimethyl formamide, N, N - amide-based solvent such as dimethylacetamide, in addition, dimethyl sulfoxide, tetrahydrofuran mixable, acetone, chloroform, methylene chloride or the like in size but, preferably aromatic solvents, aliphatic ether, aromatic ether, an aliphatic ester, aromatic ester are disclosed. They may be used alone and which 1 species, at least one retarder 2 using any combinations and proportions. (Ratio) Polymerization using the disclosure number is in the present invention, the hole-transporting compound 100 when the substituent polymerizable, 0. 01 ∼ 100 mass % is preferably, 0. 05 ∼ 50 mass % more preferably, 0. 1 ∼ 100 mass % is most preferably. 0. 01 in mass % hereinafter, polymerization and sufficiently not suitable, in 100 mass % or more, lower quality ix.. [Light-emitting layer, substrate, cathode, anode, and an electron transport layer, electron injection layer] Forming organic EL element of the present invention number 2 aspects of, light emitting layer, substrate, cathode, anode, and an electron transport layer, electron injection layer and the aforementioned number 1 and sun equal, in preferred embodiments the center of gravity of number 1 and sun or forming method are the same. Thus, in one aspect the aforementioned number 1, light emitting layer, substrate, cathode, anode, and an electron transport layer, an electron injection layer and description, in one embodiment number 2 as received signals. [Thin film forming method] Number 1 and number 2 aspects of organic EL element more, various layer in order to, for example, solution including organic electronic material, ink-jet method, casting process, the dipping law, convex plate printing, is formed on a printing, offset printing, lithography, is formed on a shift offset printing, screen printing, gravure printing mode printing, of publicly known method such as spin coating applied on a base number can be formed by coating applicator used to apply high pressure liquid coolant. In addition, thin film coating method such as the aforementioned high pressure liquid coolant light irradiation or heating treatment number polymerized by means reaction to proceed the, overlaying layer capable of solubility change (curing). Is formed by coating technique by repetition operations such as organic EL element multi-layer reduced is equal to or higher. The coating method such as described above, typically, at a temperature ranging from -20 ∼ + 300 °C, preferably 10 ∼ 100 °C, in particular preferably in embodiment 15 ∼ 50 °C can. In addition, light irradiation is said, low pressure is applied, electrolyte cathode ray tube, high-pressure mercury, super high pressure mercury lamp, halide lamp, xenon lamp, fluorescent lamp, light emitting diode, can be uses a light source such as solar. In addition, said heating processing, hot plate which can be attached to either in bake oven, at a temperature ranging from 0 ∼ + 300 °C, preferably 20 ∼ 250 °C, more preferably 50 ∼ 200 °C, most preferably 70 ∼ 150 °C can in embodiment. In low temperature sufficient curing reaction cannot be performed, and the first door number thereby residual solvent, organic EL for command thereto. In high temperature, high pressure liquid coolant it is difficult for an organic EL element number resin substrate to be coated. In addition, coating of the curing reaction, only the heat treatment, preferably for organic EL for prolonged service. [Light emitting element] Organic EL element of the present invention color are specially which are not limited to, white light emitting device home lighting, lighting in the vehicle, such as the luminaire's liquid crystal backlight can be used preferably. White light emitting device forming method uses, so far single material represent white emission connecting structure, a plurality of color of light emitted from a plurality of light emitting material of a color filter (colors) of light emitting diodes etc. by simultaneously obtaining white. As the color of light emitted from a plurality of forms, which are not specifically limited to, blue, green, red light of wavelength at 3 containing, blue and yellow, such as fairy green and orange light emitting wavelength at 2 using two complementary in size containing is cited. In addition, the f-number of color of light, can be carried out by applying a adjusts the amount to odd pixels of phosphor material. <Display device, lighting device, display device> The electroluminescent display of the present invention, the aforementioned organic EL element of the present invention etc. in the range of 0.1. For example, the, rust, blue (RGB) corresponding to each pixel of a granular, organic EL element of the present invention by using, a color display element can be achieved. Image is formed, the heated electrodes arranged in a matrix arranged individual organic EL element is disposed on the thin film transistor matrix directly with elder brother each device be elder brother active matrix driving. The electronic (former), structure of the displaying of the character is connected a simple but a vertical pixel number limit less than 2000. The latter (latter), current is filled in the low driving voltage, high precision Image light up, are used as high-quality display. In addition, illumination device of the present invention is, the aforementioned organic EL element of the present invention etc. in the range of 0.1. In addition, the device of the present invention display, on illumination device, or a liquid crystal element display means etc.. (White luminescent light source) of the present invention using the aforementioned as backlight illumination device, liquid crystal display device using the display unit device, i.e. liquid crystal display device to a retarder. This configuration, in publicly known liquid crystal display device, a backlight illumination device of the present invention only and a further pick-up device, liquid crystal device portion is dedicated ([...]) can be publicly known techniques. In the embodiment In hereinafter, more specifically described in the embodiment but by the present invention, in the embodiment of the present invention refers to hereinafter is defined as are not correct. [In the embodiment 1] (Polymerizable evaluation) The compound of the 1 (4. 5 mg) a toluene solution of (400 micro l) and lithium ion compound 1 (0. 45g) ethyl acetic acid solution (100 micro l) coating solution mixing, 3000 rpm spin-zero plate-gate on the stone. Then, in the hot plate, heated to 120 °C 10 minutes was performed. After heating toluene: hops (4:1) 1 minutes immersion ([...]) and zero plate at a lower stone, cleaning was performed. In absorbance of UV-a vis absorption spectrum before cleaning (λ max) ratio of (Abs) from, prevent precipitation were measured. Before cleaning: λ max=383 nm, Abs=0. 229 After cleaning: λ max=383 nm, Abs=0. 228 The group consisting of a Abs × 100 (%)=Abs/before cleaning after cleaning =0. 228/0. 229 × 100=99. 6 [Formula 26] [Formula 27] [In the embodiment 2] In point except the heating temperature to the hot plate 180 °C, both to prevent precipitation during the same method in the embodiment 1 were measured. [In the embodiment 3] Ion compound 1 instead, using for ion compound 2 except the point, both in the embodiment 1 to prevent precipitation during the same method were measured. [Formula 28] [In the embodiment 4] In point except the heating temperature to the hot plate 180 °C, both to prevent precipitation during the same method in the embodiment 3 were measured. [Comparison example 1] Ion compound 1 instead, using for ion compound 3 except the point, both in the embodiment 1 to prevent precipitation during the same method were measured. [Formula 29] [Comparison example 2] In point except the heating temperature to the hot plate 180 °C, comparison example 1 precipitation during the same method to both were measured. [Comparison example 3] Ion compound 1 instead, using for ion compound 4 except the point, both in the embodiment 1 to prevent precipitation during the same method were measured. [Formula 30] [Comparison example 4] In point except the heating temperature to the hot plate 180 °C, example 3 compared both to prevent precipitation during the same method were measured. [Comparison example 5] Ion compound 1 instead, using for ion compound 5 except the point, both in the embodiment 1 to prevent precipitation during the same method were measured. [Formula 31] [Comparison example 6] In point except the heating temperature to the hot plate 180 °C, compared both to prevent precipitation method identical to the example 5 were measured. Table 1 to each ion compound, 120 °C and 180 °C precipitation during evaluation results in shop window. The present invention according to ion compounds when used, than the number of the existing method using five [nyum salts style cured, is at a low temperature can be an indirectly-progressing. [Table 1] [In the embodiment 5] A ITO 1. 6 mm width mask on a glass substrate, compound 1 (4. 5 mg) a toluene solution of (400 micro l) and ion compound 1 (0. 45g) ethyl acetic acid solution (100 micro l) mixed with the coating solution, a glass substrate 3000 rpm spin-on-gate. Subsequent experiments was conducted under a dry nitrogen environment. Then, 10 minutes to 180 °C in heating the hot plate cured to, hole injection layer (40 nm) came into. Then, on the hole injection layer, a polymer to structural formula 1 (75 parts by mass), polymer 2 (20 parts by mass), polymer 3 (5 parts by mass) composed of a mixture, a toluene solution (1. 0 mass %) 3000 rpm and a spin coat, on hot plate 80 °C, heating 5 minutes, a light-emitting layer (film thickness 80 nm) weight percent. And, do not dissolve each other and been deposit hole injection layer the light emitting layer. [Formula 32] (N is, 1 or more integer equal) In addition, obtained and transferred into a methane gas glass substrate, a light emitting layer on said Ba (3 nm film thickness), Al (film thickness 100 nm) electrode in turn weight percent. Is formed on, without photo management, moving the substrate during a dry nitrogen environment, 0. 7 mm 0 of alkali-free glass. (Seal) of the countersink (countersink) 4 mm sealing glass forming ITO glass substrate patterning, photocurable epoxy resin is used to numbers of sealing is carried out, a multilayer structure of polymer type organic EL element number was high pressure liquid coolant. Subsequent experiments atmosphere, room temperature (25 °C) at him. The organic EL for ITO anodic, Al by adding a voltage applying bar, about 3V been an external view in green luminescent. 5000 cd/m luminance2 The current efficiency in 9. 1 cd/A, driving voltage is 4. 9V been. In addition, life as a characteristic, current density 13 mA/cm2 By applying a constant current ([...]), luminance halving time measuring bar, been time 340. [Comparison example 7] Ion compound 1 except the point changing ion compound 3, both in the embodiment 5 of a liquid crystal polymer type organic EL element number his method identical to the high pressure liquid coolant. The organic EL for ITO anodic, Al by adding a voltage applying bar, about 3. 5V been an external view in green luminescent. 5000 cd/m luminance2 The current efficiency in 6. 9 cd/A, driving voltage is 5. 9V been. In addition, life as a characteristic, current density 14 mA/cm2 By applying a current of, luminance halving time measuring bar, 70 been time. As compared to in the embodiment 5, high driving voltage, luminance than halving time was short. [In the embodiment 6] (organic EL characteristic of the device) Organic EL for number bath A ITO 1. 6 mm width mask on a glass substrate, a compound represented by formula 1 (3. 9 mg) compound 2 [0. 6 mg, weight average molecular weight (Mw) 247,000 Mw/Mn=1. 65, the Mn, represents a number average molecular weight] a toluene (1. 2 ml) dissolving, a group represented by the formula for polymerization disclosure number (0. 45 mg) hops a (100 micro l) dissolving, mixing them the coating solution, after 3000 rpm spin court grudge, on hot plate 180 °C, cured to 10 minutes heating, hole injection layer (30 nm) came into. [Formula 33] [Formula 34] In addition, the compound of the 3 (4. 5 mg, weight average molecular weight of 5,700 Mw/Mn=1. 94) is toluene (1. 2 ml) dissolving, said polymerization disclosure number (0. 45 mg) hops a (100 micro l) dissolving, mixing them the coating solution, after 3000 rpm spin court grudge, on hot plate 180 °C, cured to 10 minutes heating, hole transport layer (30 nm) came into. [Formula 35] Then, transferred into a methane gas glass substrate obtained and, CBP + Ir (piq)3 (40 nm), BAlq (10 nm), Alq3 (30 nm), LiF (film thickness 0. 5 nm), Al (film thickness 100 nm) light emitting layer deposited of weight percent. CBP: 4, 4' - bis (carbazole - 9 - yl) - biphenyl Ir (piq)3 : Tris (1 - phenyl isoquinoline) iridium BAlq: bis (2 - methyl - 8 - hydroxy hour [khwi[khwi] it teases but the toe) (4 - non-phenyl jade brush nato) aluminum Alq3 : Aluminum complex (tris (8 - hydroxy hour [khwi[khwi] it teases but the toe) aluminum (III)) Is formed on, without photo management, moving the substrate during a dry nitrogen environment, 0. 7 mm 0 of alkali-free glass. The countersink of 4 mm sealing glass and ITO substrate form, photocurable epoxy resin is used to numbers of sealing is carried out, a multilayer structure of polymer type organic EL element number was high pressure liquid coolant. The subsequent to the atmosphere, room temperature (25 °C) at him. The organic EL for ITO anodic, Al by adding a voltage applying bar, and emits the observed in about 4V, 1000 cd/m luminance2 The current efficiency in 7. 2 cd/A been. And, the current voltage characteristic and a current meter 4140B Hewlett Packard yarn measured fine article number, number the center of luminance-based [phu richard 1980B were measured using light emitting luminance photo it stands homicide. In addition, life as a characteristic, and a second tower cone company number the center of BM-a 7 measured while applying a constant current, the brightness is initial luminance (1000 cd/m2 ) And measuring the time for halving from above, been time 270. (In the embodiment 6 of the stack of confirmation) In the embodiment 6 of a quartz changing point except the ITO, the hole injection layer all have a same method weight percent. Hole injection layer 1 minutes to be dipped in toluene, toluene immersion before absorption spectrum of intensity variations as observing, 99. 8% knew which controls the operating temperature. On the hole injection layer, a hole transporting as well as in the embodiment 1 weight percent. Hole transport layer 1 minutes to be dipped in toluene, toluene immersion before absorption spectrum of intensity variations as observing, 97. Which controls was 8%. [In the embodiment 7] In in the embodiment 6, hole transporting layer, polymerization disclosure number (0. 45 mg) is added to a point other than organic EL element formed without any high pressure liquid coolant number was the same method. The organic EL for ITO anodic, Al by adding a voltage applying bar, about 3. 5V and emits in observed, 1000 cd/m luminance2 The current efficiency in 8. 4 cd/A been. In addition, initial luminance brightness (1000 cd/m2 ) And measuring the time for halving from above, been time 1020. (In the embodiment 7 of the stack of confirmation) Changing a point of quartz in the embodiment 7 except the ITO, the hole injection layer all have a same method weight percent. Hole injection layer 1 minutes to be dipped in toluene, toluene immersion before absorption spectrum of intensity variations as observing, 99. 8% knew which controls the operating temperature. On the hole injection layer, hole transport in the same manner as in the embodiment 2 on weight percent. Hole transport layer 1 minutes to be dipped in toluene, toluene immersion before absorption spectrum of intensity variations as observing, 97. Which controls was 2%. [Comparison example 8] In the embodiment 6 1 2 instead of compound compound in compound 2 (4. 5 mg) is inputted, the hole injection layer is a mixed solution polymerization disclosure number crude number (30 nm) is formed except the point, the same method in the embodiment 6 organic EL element number was high pressure liquid coolant. The organic EL for ITO anodic, Al by adding a voltage applying bar, emits fluorescence observed in about 6V, 1000 cd/m luminance2 In the current efficiency 1. 3 cd/A been. In addition, initial luminance brightness (1000 cd/m2 ) And measuring the time for halving from above, been time 12. (Comparison example 8 of the stack of confirmation) Comparison example 8 except the point of a quartz changing ITO, the hole injection layer all have a same method weight percent. Hole injection layer 1 minutes to be dipped in toluene, toluene immersion absorption spectrum of intensity variation as observed before, 2. 8% without a membrane is placed which brings out, stacks number could not be high pressure liquid coolant. 8 adjacent such as comparison example 2 in dog layer, the hole-transporting compound each polymerizable substituent including mixture if not, number which does not laminated structure including a high pressure liquid coolant, luminous efficiency, minute superfine life but, as shown in the embodiment 6, 7, adjacent 2 in dog layer, the hole-transporting compound by using each polymerizable substituent including when, hereinafter for laminated structure can be formed, luminous efficiency, excellent element life can be achieved. In addition, such as in the embodiment 7, close to the anode of the light mixing layer polymer disclosure number only, excellent life number can be the liquid high pressure liquid coolant. [In the embodiment 8] A ITO 1. 6 mm width mask on a glass substrate, a compound represented by the formula 4 (4. 5 mg, Mw=7,700, Mw/Mn=1. 45) a toluene (1. 2 ml) dissolving, said polymerization disclosure number (0. 45 mg) hops a (100 micro l) dissolving, mixing them the coating solution, after 3000 rpm spin court grudge, on hot plate 180 °C, cured to 10 minutes heating, hole injection layer (30 nm) came into. [Formula 36] Then, compounds represented by the following formula on the hole injection layer 5 (4. 5 mg, Mw=10,800, Mw/Mn=1. 52), toluene (1. 2 ml) mixed with the coating solution, after 3000 rpm spin court grudge, on hot plate 180 °C, cured to 10 minutes heating, hole transport layer (30 nm) came into. [Formula 37] Then, hole transporting layer, a polymer to structural formula 1 (75 parts by mass), polymer 2 (20 parts by mass), polymer 3 (5 parts by mass) composed of a mixture, a toluene solution (1. 0 mass %) 3000 rpm and a spin coat, on hot plate 80 °C, heating 5 minutes, a light-emitting layer (film thickness 80 nm) weight percent. And, hole transporting layer the light emitting layer can be laminated to each other without been dissolved. In addition, obtained and transferred into a methane gas glass substrate, a light emitting layer on said Ba (3 nm film thickness), Al (film thickness 100 nm) electrode in turn weight percent. Is formed on, without photo management, moving the substrate during a dry nitrogen environment, 0. 7 mm 0 of alkali-free glass. The countersink of 4 mm sealing glass and ITO substrate form, photocurable epoxy resin is used to numbers of sealing is carried out, a multilayer structure of polymer type organic EL element number was high pressure liquid coolant. The subsequent to the atmosphere, room temperature (25 °C) at him. The organic EL for ITO anodic, Al by adding a voltage applying bar, about 3. 5V been an external view in green luminescent. 5000 cd/m luminance2 The current efficiency in 8. 1 cd/A, driving voltage is 6. 1V been. In addition, initial luminance brightness (1000 cd/m2 ) And measuring the time for halving from above, time been 990. [Formula 38] 1: light emitting layer 2: anode 3: hole injection layer 4: cathode 5: the metal layer 6: hole transport layer 7: electron transport layer 8: substrate Provided is a material for organic electronics which can produce an organic electronic element capable of lowering the driving voltage or capable of performing stable driving for a long time. The material for organic electronics contains at least an ionic compound and a compound having a charge transporting unit (hereinafter, referred to as charge transporting compound), and the ionic compound is composed of a counter cation and a counter anion, while the counter cation is any one kind or two or more kinds selected from H + , a carbocation, a nitrogen cation, an oxygen cation, and a cation having a transition metal. Multilayer structure is organic electro-light-emitting device as number bath method, said multilayer structure (a) and (b) either dog layer adjacent steps 2 through number bath and, the hole-transporting compound (a) polymerizable substituent after mixture is applied onto a substrate including, forming curing dog layer 1, and (b) formed on said wustite 1, the hole-transporting compound polymerizable substituent including after applying the mixture, forming other curing 1 dog layer, said step (a) and step (b) at least in one step, hardening made by the above method, said polymerizable substituents at the probe and an oxetane, 2 during wustite layer adjacent said, anode layer near disclosure number only mixing polymer, said polymer is disclosure number, carbon cation, nitrogen cation and acid cations is used cations selected from the group consisting having ion compound, organic electro-light-emitting device number bath method. Back number According to Claim 1, said hole transporting compound, at least arylamine, carbazole, including thiophene skeleton, organic electro-light-emitting device number bath method. According to Claim 1, said hole transporting compound, number average molecular weight of 1000 or more oligomer or polymer, organic electro-light-emitting device number bath method. Back number Back number According to Claim 1, said ion compound and an electron-accepting compound, said hole transport compound 1 is an electronic unoxidized, organic electro-light-emitting device number bath method. According to Claim 1, a plurality of luminous organic electro-said this resin film, organic electro-light-emitting device number bath method. According to Claim 1, performed at a temperature ranging from - 20 - + 300 °C application said, organic electro-light-emitting device number bath method. Back number