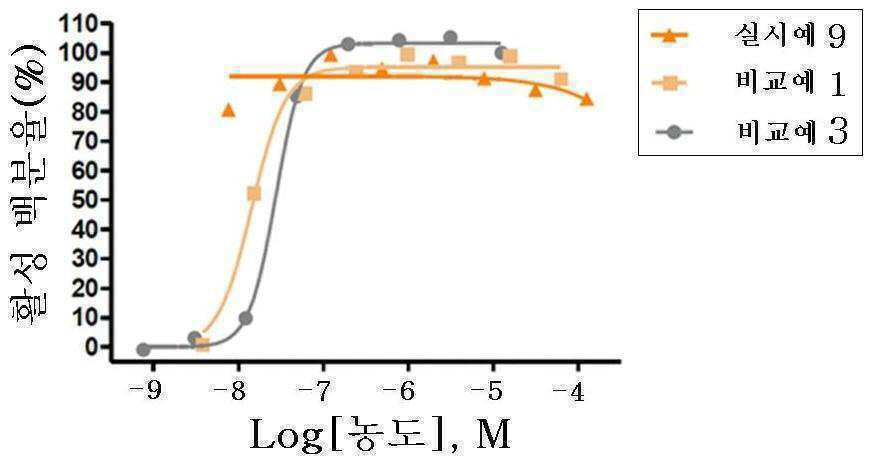
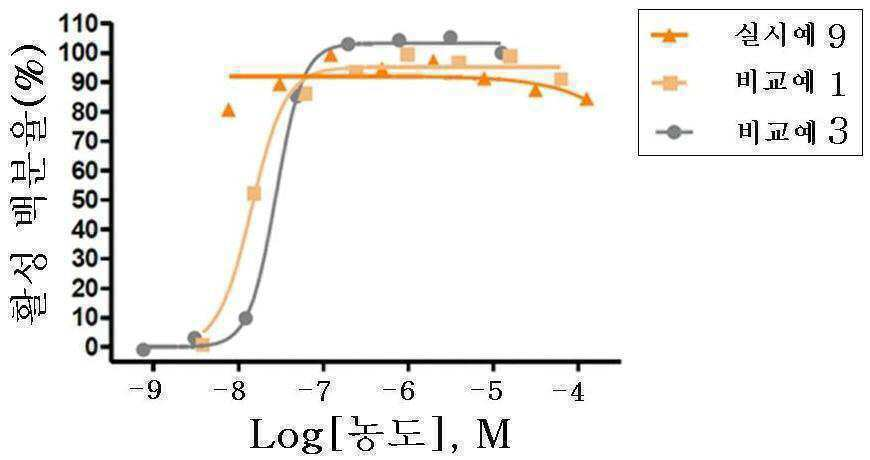
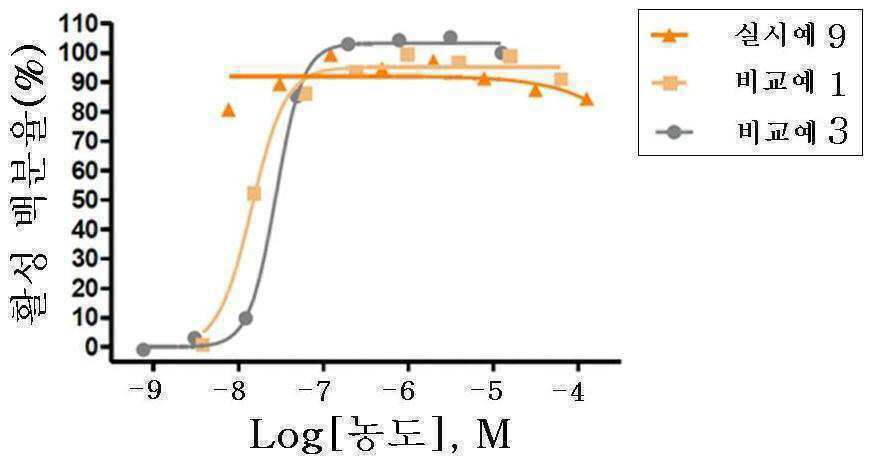
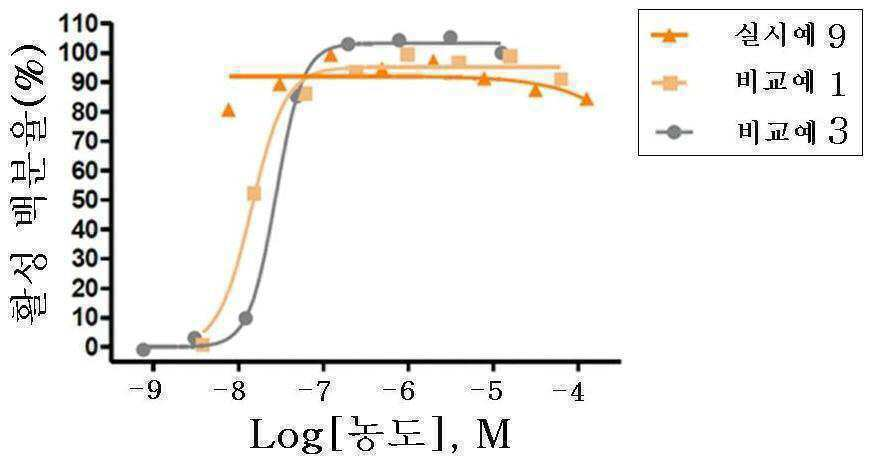
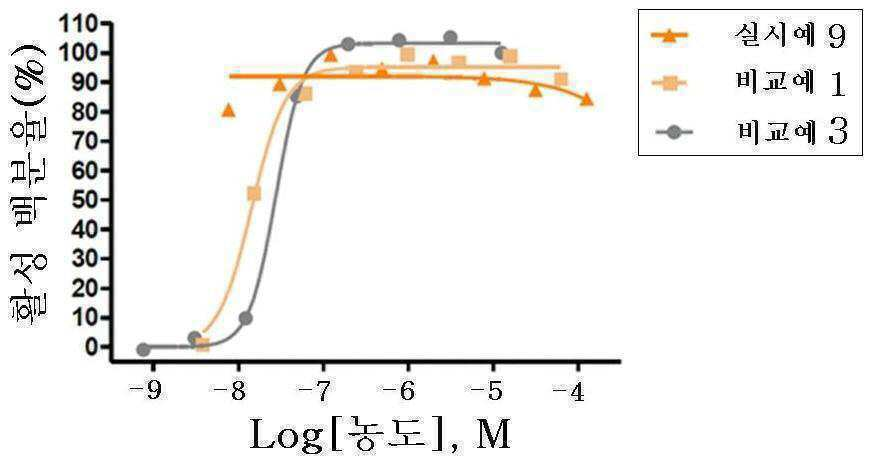
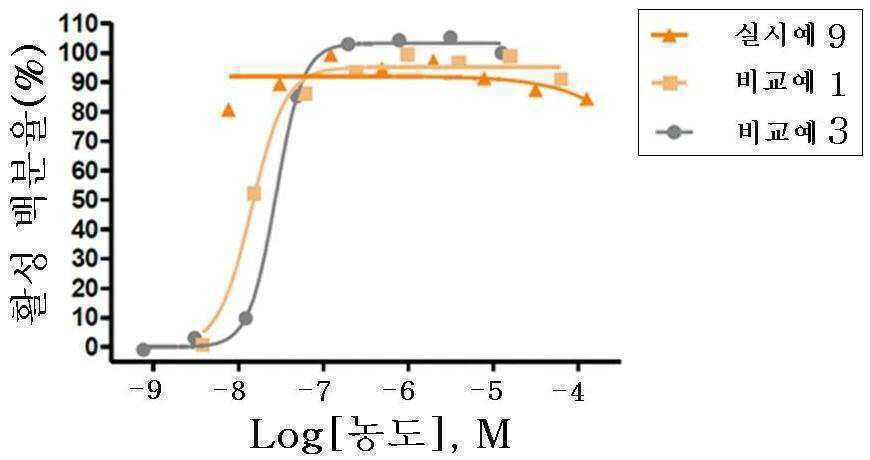
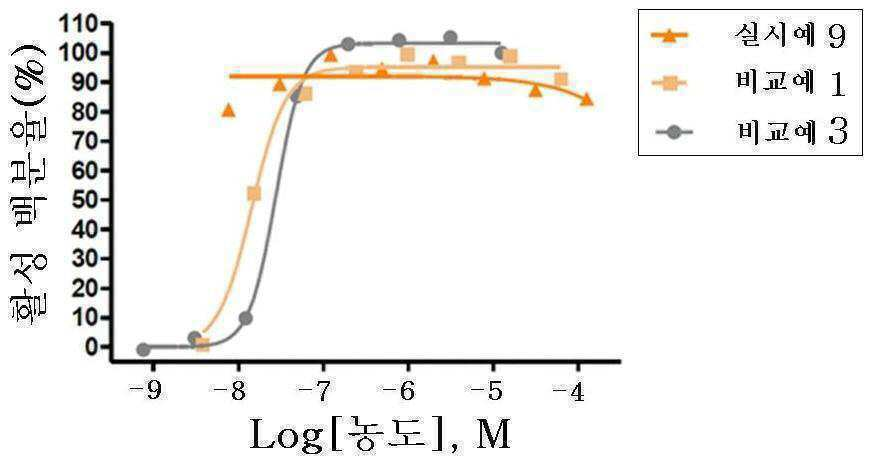
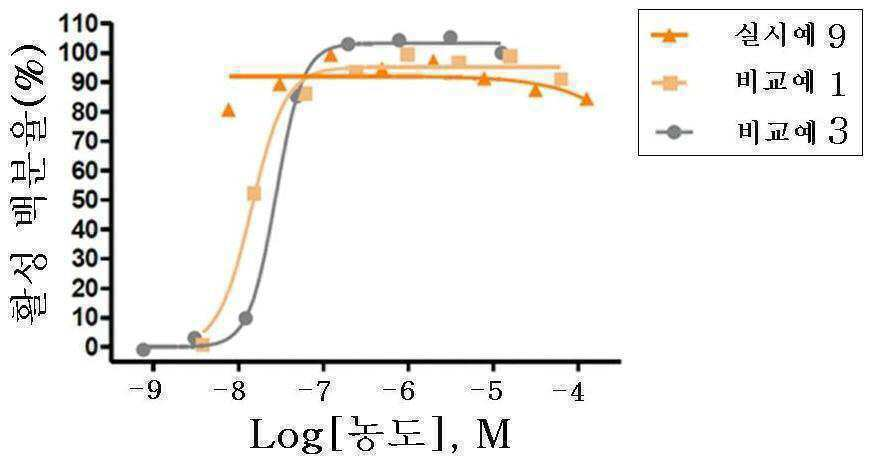
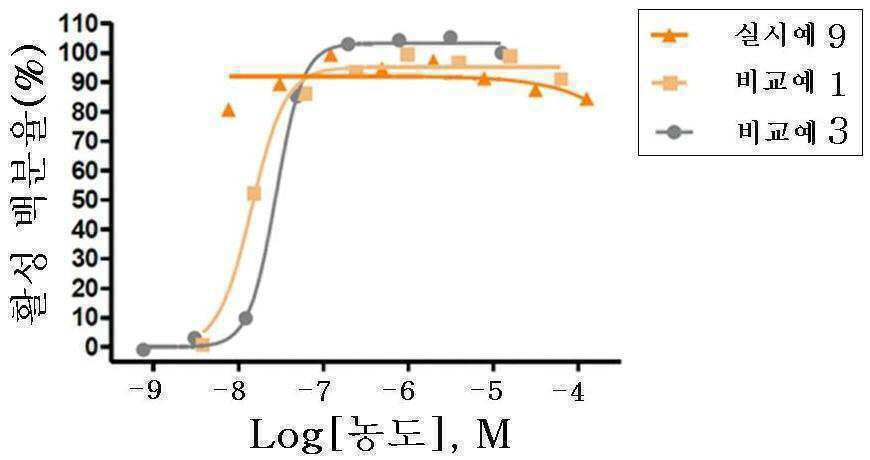
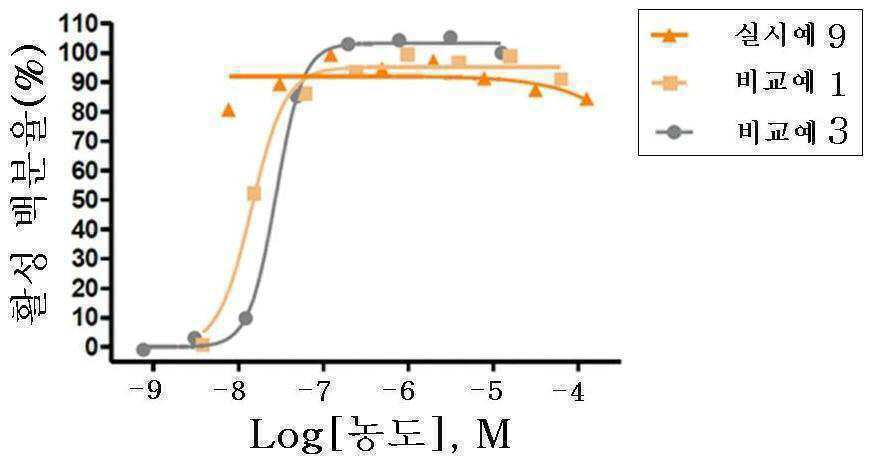
신규한 3-(4-(벤질옥시)페닐)헥스-4-이노익 산 유도체 및 다른 유효성분을 포함하는 대사성 질환의 예방 또는 치료용 복합제제
The present invention relates to novel 3 - (4 - (benzyloxy) phenyl) - 4 - wing acid derivatives IV d [pheyp[pheyp] mote [til[til] mote day [cu[cu][heyk[heyk] inosinate (Dipeptidyl peptidase-a 4, DPPIV) inhibitors based, sulfonylureas (Sulfonylurea) sequence, tooth it will doze [tin[tin] D it comes (Thiazolidinediones, TZD) sequence, and SGLT2 (sodium/glucose cotransporter 2) inhibitor sequence-pharmaceutical (Biguanide) sequence and at least one other active drug selected from a group consisting of a combination can be administered in combination or in the form of a pharmaceutical composition for prophylaxis or treatment of metabolic diseases are disclosed. Name of greater than 1 billion people suffering from diabetes is injected on aero-severe disease, etc. continuously threatening human health. The I and II type 2 diabetes can be classified into two clinical syndrome type. Insulin dependent diabetes (IDDM) diabetes of type I known even insulin producing pancreatic beta cells derived by exogenous insulin administration of autoimmune and in which require routine. Diabetes of type II non-insulin dependent diabetes (NIDDM) known hypoglycemic levels even properly controlled by the loss of its capability shown substrate. Diabetes of type II defects in insulin secretion or insulin resistance (insulin resistance), i.e. insulin or no insulin cannot be used effectively in people having diabetes type II can be characterized by a. On the other hand, blood and urine glucose is high value and accumulated in diabetes, the resulting excessive urination, thirst, hunger, fat and lipoprotein metabolism problems associated with deformable cladding layer. Boiled to take threatening complications, e.g. visual acuity loss, causing venous heart disease can be elongated, and the backside of the eye retina causes damage, enhance the risk of cataract and glaucoma. Also, legs and feet of neuronal damage with respect to the ability to feel pain as the, a common cause of serious infections of the tape substrate. Conventional, to the use of currently treatment with insulin, insulin secretagogue, glucose lowering effector (effector), activators for peroxisome proliferator activated receptor (PPAR) user - etc.. However, blood, body weight gain, for the treatment of reactive reduced time elapses, edema and gastrointestinal problems associated with bisphosphonate therapies currently available to be problem. In accordance with more efficient new therapy to introduce a targeted market studies and various areas is not, one particular as targets G protein coupled receptor (G non-protein coupled receptor: GPCR) studies of progressing disclosed. Recently, it has been one of GPR40 G protein coupled receptor (G non-protein coupled receptor: GPCR). This overexpression of β cells of the pancreas is known as free fatty receptor 1 (free fatty acid receptor 1) being disclosed. GPR 40 (FFAR1) activating the compound an intracellular calcium concentration is increased, the glucose - stimulating insulin secretion (glucose non-stimulated insulin secretion, GSIS) (non-Patent document 1) a known to promote. Also, due to the diabetic and diabetic-prone mouse comprises a normal mouse or genetic mutations GPR40 activator administered before testing glucose intolerance, impaired are improved inhibin receptor. In these processed mouse plasma insulin levels of short increased also inhibin receptor. GPR40 function with respect to GPR40 ligand free fatty acid is by the effect of pancreatic β cells, glucose in dependence on insulin secretion β cells in known. Also, GPR knockout mouse (knockout) analysis of the GPR 40 can be involved in the pathology of diabetes obesity and (non-Patent document 2) at or. For this reason GPR 40 new diabetes etc. target genes of interest. The, the present inventor has discovered novel 3 - (4 - (benzyloxy) phenyl) - 4 - wing acid derivatives IV d [pheyp[pheyp] mote [til[til] mote day [cu[cu][heyk[heyk] inosinate (Dipeptidyl peptidase-a 4, DPPIV) inhibitors based, sulfonylureas (Sulfonylurea) sequence, tooth it will doze [tin[tin] D it comes (Thiazolidinediones, TZD) series, and SGLT2 (sodium/glucose cotransporter 2) inhibitor-based drug-pharmaceutical (Biguanide) sequence selected from the group consisting of at least one other electro active combination, making sure that the excellent fall effect level representing the instant invention the arrears of work. A plurality of papers Patent document reference herein throughout its cited displayed disclosed. Reference herein in their entirety upon ejected Patent document disclosure provides an as is inserted into this invention is in the field of the present invention and the level of the content of the more specifically described. The aim of the invention is novel 3 - (4 - (benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing acid derivatives, their isomers, hydrates, solvates, or pharmaceutically acceptable salt thereof d [pheyp[pheyp] mote [til[til] mote day [cu[cu] IV (Dipeptidyl peptidase-a 4, DPPIV) inhibitors based, sulfonylureas (Sulfonylurea) sequence, tooth it will doze [tin[tin] D it comes (Thiazolidinediones, TZD) sequence, and SGLT2 (sodium/glucose cotransporter 2) inhibitors (Biguanide) sequence-pharmaceutical drug-selected from a group consisting of at least one other active complex can be administered in combination or in the form of a pharmaceutical composition for prophylaxis or treatment of metabolic diseases are disclosed. The advantage of and another object of the invention to form a description, claims and drawings by more specifically with each other. In order to attain the object, the present invention relates to compounds of formula 1 as first effective 1 (a), their isomers, hydrates, solvates or pharmaceutically acceptable salts thereof and (b) 2 (Dipeptidyl peptidase-a 4) inhibitors as first effective d [pheyp[pheyp] mote [til[til] mote day [cu[cu] IV family, sulfonylureas (Sulfonylurea) sequence, tooth it will doze [tin[tin] D it comes (Thiazolidinediones) sequence, and SGLT2 (sodium/glucose cotransporter 2) inhibitor compounds-pharmaceutical (Biguanide) sequence selected from the group consisting of one or more compositions for preventing or treating metabolic disorders comprising: [Formula 1] (In the formula 1, A single bond, or a double bond and; A and E are independently C, N, or O and; And n is 0 - 5; X is single bond, or C1-10 Straight or branched alkyl [leyn[leyn] And; R1 Is - H, - OH, halogen, C1 -10 A straight or branched alkyl, C1-10 A straight or branched alkoxy, C5-10 Of cycloalkyl, or C5-10 Preparation of a 10,000; R2 , R 3 , And R5 Are independently - H, - OH, halogen, C1-10 A straight or branched alkyl, or C1-10 A straight or branched alkoxy and; Wherein, the R2 And R3 C atomic connected together with these5-10 Of cycloalkyl, C6 - 10 Aryl, N, O and S heteroatoms selected from the group consisting of 5 - 10 atoms comprising at least one hetero cycloalkyl, or N, O and S it will be biting hetero atoms selected from the group consisting of 5 - 10 can be formed comprising at least one hetero atoms; R4A Is - H, - OH, =O, unsubstituted or substituted C6-10 Aryl, or N, O and S heteroatoms selected from the group consisting of unsubstituted or substituted C comprising at least one5 - 10it will be biting and heteroatoms, Wherein, the substituted C6-10 Aryl, substituted C5-10it will be biting heteroatoms independently - OH, halogen, knight reel, unsubstituted or one or more substituted with halogen C1-5 Straight or branched alkyl, unsubstituted or substituted with one or more halogens C1-5 Straight or branched alkoxy, C1 - 10 A straight or branched alkyl methylsulfonyl, , And At least one substituent selected from the group consisting of one or more can be substituted 1, wherein, the integer number of m and q are independently 1 - 10, Also, the non-substituted or substituted C5-10 Of heteroaryl is phenyl the fusion (fused) can be; Wherein, the R3 And R4A C atomic connected together with these5-10 Of cycloalkyl, C6-10 Aryl, N, O and S heteroatoms selected from the group consisting of 5 - 10 atoms comprising at least one hetero cycloalkyl, or N, O and S it will be biting hetero atoms selected from the group consisting of 5 - 10 can be formed comprising at least one hetero atoms, Also, the C5-10 Of cycloalkyl, C6-10 Aryl, 5 - 10 atoms hetero cycloalkyl, and 5 - 10 atom heteroaryl heats the C1-5 Straight or branched alkoxy can be substituted; R4B The member or, or R4B The R4B Connected atom and R4A N with, comprising at least one hetero atoms selected from the group consisting of O and S C5-10 Heterocycles can be formed. Wherein, the d [pheyp[pheyp] mote [til[til] mote day [cu[cu] IV (Dipeptidyl peptidase-a 4, DPPIV) inhibitor sequence is at the time of hit writing [lip[lip] (Sitagliptin), billets it will bring near, [lip[lip] (Vildagliptin), [sak[sak] It buys the writing [lip[lip] (Saxagliptin), contamination in writing [lip[lip] (Linagliptin), reel four writing [lip[lip] (Teneligliptin), with egg writing [lip[lip] (Alogliptin), my Americawriting [lip[lip] (Gemigliptin), two toggles [lip[lip] (Dutogliptin), base (Berberine), loupe this year (Lupeol), red [ayl[ayl] Compared to (red alder (Alnus rubra)), and which is essentially it is burnt it comes only coffee (dandelion coffee), sulfonylureas (Sulfonylurea) examples of polymerization-polyimide (Carbutamide), [heyk[heyk] It buys America [tu[tu] acetoacetoxy-functional (Acetohexamide), claw [lu[lu] pro group America [tu[tu] (Chlorpropamide), [thol[thol] Father hit America [tu[tu] (Tolbutamide), writing pizza id (Glipizide), gliclazide (Gliclazide), writing it cuts the lama id which will grow (Glibenclamide), writing step [lu[lu] world [tu[tu] (Glibornuride), writing [khwi[khwi] money (Gliquidone), writing small storms of life id (Glisoxepide), selected from the group consisting of 1 species writing nose bleed America [tu[tu] (Glyclopyramide) and glimepiride (Glimepiride) and; The tooth it will doze [tin[tin] D it comes (Thiazolidinediones, TZD) sequence is (Rosiglitazone) rosiglitazone, pioglitazone (Pioglitazone), of preventing (Troglitazone), four toggle it burns the zone (Netoglitazone), rosiglitazone (Rivoglitazone) ribonucleotides, hour writing it burns the zone (Ciglitazone), metformin (Metformin) is a rhodanine (Rhodanine) at sea-based pharmaceutical, the pen gun it pushed (Phenformin), the vice-pawl it pushed (Buformin), pro it will not be nine (Proguanil), claw [lu[lu] pro nine will not be (Chlorproguanil), chlorhexidine (Chlorhexidine), polyamino profile pharmaceutical (Polyaminopropyl biguanide (PAPB)), selected from the group consisting of poly (Polihexanide) and the rack which will knowat the time of [tin[tin][heyk[heyk] San'A id (Alexidine) and 1 species; The SGLT2 inhibitor compounds (sodium/glucose cotransporter 2) the emphasis glycol ripple rosin (empagliflozin), selected from the group consisting 1 (dapagliflozin) (canagliflozin) and multi green onion writing ripple rosincar orwriting ripple rosin paper disclosed. The novel 3 - (4 - (benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing acid derivatives, their isomers, hydrates, solvates, or pharmaceutically acceptable salt thereof d [pheyp[pheyp] mote [til[til] mote day [cu[cu] IV (Dipeptidyl peptidase-a 4, DPPIV) inhibitors based, sulfonylureas (Sulfonylurea) sequence, tooth it will doze [tin[tin] D it comes (Thiazolidinediones, TZD) sequence, and SGLT2 (sodium/glucose cotransporter 2) inhibitor sequence-pharmaceutical (Biguanide) sequence and at least one other active drug from the group consisting of selecting the method result, exhibit excellent blood glucose fall effect various animal disease model Image are diabetes, said derivatives, their isomers, hydrates, solvates, or pharmaceutically acceptable salt thereof d [pheyp[pheyp] mote [til[til] mote day [cu[cu] IV (Dipeptidyl peptidase-a 4, DPPIV) inhibitors based, sulfonylureas (Sulfonylurea) sequence, tooth it will doze [tin[tin] D it comes (Thiazolidinediones, TZD) sequence, and SGLT2 (sodium/glucose cotransporter 2) inhibitors (Biguanide) sequence-pharmaceutical drug from the group consisting of one or more other less-active form by selecting a combination can be administered in combination or obesity, diabetes of type I, diabetes type II, coincidental glucagon-like peptide, insulin resistance, hyperglycemia, aims, high the Holy Land bloodletting symptoms, hypercholesterolemia or mixed dyslipidemia, hypertriglyceridemia, such as sick X for useful pharmaceutical compositions for preventing or treating metabolic disorders can be. Figure 1 shows a concentration of compounds 9 and comparison example 1, 3 also embodiments according to positive measuring graph and GPR40 protein activation; Figure 2 shows a SD (Sprague Dawley rat) compounds and comparison example 1 to 9 also for detecting BAX embodiments oral-compounds, blood GLP-a 1 indicating graph livestock products are disclosed. Figure 3 dietary obesity model (Diet a-Induced Obesity, DIO) mouse embodiments 9 compounds, or at the time of hit writing [lip[lip]at the time of hit writing [lip[lip] administered alone or embodiments as to hypoglycemic (%) represents a 9 compound and the administration of a combination are disclosed. Figure 4 dietary obesity model (Diet a-Induced Obesity, DIO) mouse embodiments 9 compounds, or writing maul blood leadingwriting maul blood leading administered alone or embodiments as to hypoglycemic (%) represents a 9 compound and the administration of a combination are disclosed. Figure 5 dietary obesity model (Diet a-Induced Obesity, DIO) mouse embodiments 9 compound, rosiglitazone, pioglitazone or rosiglitazone writing it burns the zone 9 compound and administered alone or embodiments, or embodiments 9 writing it burns the zone pioglitazone (%) represents a hypoglycemic compound as to the administration of a combination are disclosed. Figure 6 to 9 (Zucker Diabetic Fatty, ZDF) embodiments for detecting BAX the [cyu[cyu] it is big diabetic fat excessive model compounds, or the maul [thu[thu] gun it pushedthe maul [thu[thu] gun it pushed administered alone or embodiments as to hypoglycemic (%) represents a 9 compound and the administration of a combination are disclosed. Figure 7 SD (Sprague Dawley) (rat) for detecting BAX to embodiments 9 compound, administered alone or embodiments 9 compounds and contamination in the administration of a combination writing [lip[lip]writing [lip[lip] contamination in or as to hypoglycemic (%) represents a degree are disclosed. Figure 8 SD (Sprague Dawley) (rat) for detecting BAX to embodiments 9 compounds, or emphasis glycol or glycol compounds and emphasis the administration of a combination embodiments 9 ripple rosin administered alone as to hypoglycemic (%) represents a ripple rosin are disclosed. Figure 9 SD (Sprague Dawley) (rat) for detecting BAX to embodiments 9 compounds, or the maul [thu[thu] gun it pushedthe maul [thu[thu] gun it pushed administered alone or embodiments as to hypoglycemic (%) represents a 9 compound and the administration of a combination are disclosed. Figure 10 NCI a-H716 using the same Below, the present invention detailed as follows. According to one aspect of the present invention, the invention relates to compounds of formula 1 as first effective 1 (a), their isomers, hydrates, solvates or pharmaceutically acceptable salts thereof and (b) 2 (Dipeptidyl peptidase-a 4) inhibitors as first effective d [pheyp[pheyp] mote [til[til] mote day [cu[cu] IV family, sulfonylureas (Sulfonylurea) sequence, tooth it will doze [tin[tin] D it comes (Thiazolidinediones) sequence, and SGLT2 (sodium/glucose cotransporter 2) inhibitor compounds-pharmaceutical (Biguanide) sequence selected from the group consisting of one or more compositions for preventing or treating metabolic disorders comprising: [Formula 1] (In the formula 1, A single bond, or a double bond and; A and E are independently C, N, or O and; And n is 0 - 5; X is single bond, or C1-10 Straight or branched alkyl [leyn[leyn] And; R1 Is - H, - OH, halogen, C1-10 A straight or branched alkyl, C1-10 A straight or branched alkoxy, C5-10 Of cycloalkyl, or C5-10 Preparation of a alkenyl and; R2 , R3 , And R5 Are independently - H, - O H, halogen, C1-10 A straight or branched alkyl, or C1-10 A straight or branched alkoxy and; Wherein, the R2 And R3 C atoms and to which a shake caused by these5-10 Of cycloalkyl, C6-10 Aryl, N, O and S heteroatoms selected from the group consisting of 5 - 10 atoms comprising at least one hetero cycloalkyl, or N, O and S army [u[u]it will be biting hetero atoms selected from consisting of 5 - 10 can be formed comprising at least one hetero atoms; R4A Is - H, - OH, =O, unsubstituted or substituted C6-10 Aryl, or N, O and S heteroatoms selected from the group consisting of unsubstituted or substituted C comprising at least one5 - 10it will be biting and heteroatoms, Wherein, the substituted C6-10 Aryl, substituted C5-10it will be biting heteroatoms independently - OH, halogen, knight reel, unsubstituted or one or more substituted with halogen C1-5 Straight or branched alkyl, unsubstituted or substituted with one or more halogens C1-5 Straight or branched alkoxy, C1-10 A straight or branched alkyl methylsulfonyl, , And At least one substituent selected from the group consisting of one or more can be substituted 1, wherein, the integer number of m and q are independently 1 - 10, Also, the non-substituted or substituted C5-10 Of heteroaryl is phenyl the fusion (fused) can be; Wherein, the R3 And R4A C atomic connected together with these5-10 Of cycloalkyl, C6-10 Aryl, N, O and S heteroatoms selected from the group consisting of 5 - 10 atoms comprising at least one hetero cycloalkyl, or N, O and S it will be biting hetero atoms selected from the group consisting of 5 - 10 can be formed comprising at least one hetero atoms, Also, the C5-10 Of cycloalkyl, C6-10 Aryl, 5 - 10 atoms hetero cycloalkyl, and 5 - 10 atom heteroaryl heats the C1-5 Straight or branched alkoxy can be substituted; R4B The member or, or R4B The R 4B Connected atom and R4A N with, comprising at least one hetero atoms selected from the group consisting of O and S C5-10 Heterocycles can be formed. In one embodiment of the present invention, A A single bond, or a double bond and; A and E are independently C, N, or O and; And n is 0 - 3; X is single bond, or C1-5 Straight or branched alkyl [leyn[leyn] And; R1 Is - H, - OH, halogen, C1-5 A straight or branched alkyl, C1-5 A straight or branched alkoxy, C5-8 Of cycloalkyl, or C5-8 Preparation of a 10,000; R2 , R3 , And R5 Are independently - H, - OH, halogen, C1-5 A straight or branched alkyl, or C1-5 A straight or branched alkoxy and; Wherein, the R2 And R3 C atomic connected together with these5-8 Of cycloalkyl, C6 - 8 Aryl, N, O and S heteroatoms selected from the group consisting of hetero atoms comprising at least one 5 - 8 cycloalkyl, or N, O and S heteroatoms selected from the group consisting of comprising at least one hetero atom can form a 5 - 8 it will be biting and; R4A Is - H, - OH, =O, unsubstituted or substituted C6-8 Aryl, or N, O and S heteroatoms selected from the group consisting of unsubstituted or substituted C comprising at least one5 - 8it will be biting and heteroatoms, Wherein, the substituted C6-8 Aryl, substituted C5-8it will be biting heteroatoms independently - OH, halogen, knight reel, unsubstituted or one or more substituted with halogen C1-5 Straight or branched alkyl, unsubstituted or substituted with one or more halogens C1-5 Straight or branched alkoxy, C1-8 A straight or branched alkyl methylsulfonyl, , And At least one substituent selected from the group consisting of one or more can be substituted 1, wherein, the integer number of m and q are independently 1 - 5, Also, the non-substituted or substituted C5-8 Of heteroaryl is phenyl the fusion (fused) can be; Wherein, the R3 And R4A C atomic connected together with these5-8 Of cycloalkyl, C6-8 Aryl, N, O and S heteroatoms selected from the group consisting of hetero atoms comprising at least one 5 - 8 cycloalkyl, or N, O and S it will be biting hetero atoms selected from the group consisting of 5 - 8 can be formed comprising at least one hetero atoms, Also, the C5-8 Of cycloalkyl, C6-8 Aryl, hetero atoms 5 - 8 cycloalkyl, 5 - 8 and heats the C atom heteroaryl 1 - 5 Straight or branched alkoxy can be substituted; R4B The member or, or R4B The R4B Connected atom and R4A N with, comprising at least one hetero atoms selected from the group consisting of O and S C5-8 Heterocycles can be formed. In one embodiment aspect of the invention, A A single bond, or a double bond and; A and E are independently C or N and; And n is 0 - 1; X is single bond, or C1-3 Straight or branched alkyl [leyn[leyn] And; R1 Is - H, or And; R2 , R3 , And R5 And independently - H, Wherein, the R2 And R3 Phenyl and are joined together they form; R4A Is - H, - OH, =O, , , , , , , , , , , , Or And, Wherein, the R3 And R4A As they can be formed with connected atoms and phenyl, the phenyl is substituted maul [thok[thok] town can be; R4B The member or, or R4B The R4B Connected atom and R4A With Can be formed. In one embodiment one of the present invention, the formula 1 compound is a compound selected from the group consisting either are disclosed: (1) 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - tooth [heyk[heyk] inosinate; (2) 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - L - inosinate [heyk[heyk] tooth lysine salt; (3) 4 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - tooth [heyk[heyk] inosinate; (4) 3 - (4 - (3 - (4 - oxo - 1 - enyl gap claw [heyk[heyk]) benzyloxy) phenyl) - 4 - tooth [heyk[heyk] inosinate; (5) 3 - (4 - (3 - (4 - hydroxy - 1 - enyl current events claw [heyk[heyk]) benzyloxy) phenyl) - 4 - tooth [heyk[heyk] inosinate; (6) 3 - (4 - (3 - (4 - hydroxy - 1 - enyl current events claw [heyk[heyk]) benzyloxy) phenyl) - 4 - L - inosinate [heyk[heyk] tooth lysine salt; (7) (3S) - 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - tooth [heyk[heyk] inosinate; (8) (3R) - 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - tooth [heyk[heyk] inosinate; (9) (3S) - 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - L - inosinate [heyk[heyk] tooth lysine salt; (10) (3R) - 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - L - inosinate [heyk[heyk] tooth lysine salt; (11) (3S) - 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - tooth [heyk[heyk] inosinate sodium salt; (12) 3 - (4 - (4 - ((3, 4 - dihydroisoquinoline -2 (1H) - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (13) 3 - (4 - (3 - 1 - 4 - ((3, 4 - dihydroisoquinoline -2 (1H) - yl) methyl) fragrance-benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (14) 3 - (4 - (4 - ((4 - phenyl - 5, 6 - dihydropyridine -1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (15) 3 - (4 - (4 - ((4 - phenyl piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (16) 3 - (4 - (4 - ((6 - methoxy - 3, 4 - dihydroisoquinoline -2 (1H) - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (17) 3 - (4 - (4 - ((4 - phenylpiperidine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (18) 3 - (4 - (4 - ((4 - (4 - fluorophenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (19) 3 - (4 - (4 - ((4 - (4 - (trifluoromethyl) phenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (20) 3 - (4 - (4 - ((4 - (4 - (3 - (methylsulfonyl) propoxy) phenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (21) (S)- 3 - (4 - (4 - ((3, 4 - dihydroisoquinoline -2 (1H) - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (22) (S)- 3 - (4 - (4 - ((4 - (4 - (trifluoromethyl) phenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (23) (S)- 3 - (4 - (4 - ((4 - (4 - fluorophenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (24) potassium (S)- 3 - (4 - (4 - ((4 - (4 - (trifluoromethyl) phenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate; (25) (S)- 3 - (4 - (4 - ((6 - methoxy - 3, 4 - dihydroisoquinoline -2 (1H) - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (26) (S)- 3 - (4 - (4 - ((4 - phenylpiperidine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (27) (S)- 3 - (4 - (4 - (- 2 - isoindoline ylmethyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (28) (S)- 3 - (4 - (4 - ((4 - phenyl - 5, 6 - dihydropyridine -1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (29) (S)- 3 - (4 - (4 - ((4 - (4 - (maul [thok[thok] hour maul [thok[thok] city) phenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (30) (S)- 3 - (4 - (4 - ((4 - (5 - isopropyl - 1, 2, 4 - oxadiazole - 3 - yl) piperidine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (31) (S)- 3 - (4 - (4 - ((4 - (5 - isopropyl - 1, 2, 4 - oxadiazole - 3 - yl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (32) (S)- 3 - (4 - (4 - ((4 - (4 - (methylsulfonyl) phenyl) - 5, 6 - dihydropyridine -1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (33) (S)- 3 - (4 - (4 - ((4 - (4 - (3 - (methylsulfonyl) propoxy) phenyl) - 5, 6 - dihydropyridine -1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (34) (3S) - 3 - (4 - (4 - (1 - (3, 4 - dihydroisoquinoline -2 (1H) - yl) ethyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (35) (S)- 3 - (4 - (4 - ((4 - (4 - hydroxyphenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (36) (S)- 3 - (4 - (4 - ((4 - (4 - (3 - (methylsulfonyl) propoxy) phenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (37) sodium (S)- 3 - (4 - (4 - (- 2 - isoindoline ylmethyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate; (38) L - lysine (S)- 3 - (4 - (4 - (- 2 - isoindoline ylmethyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate; (39) (S)- 3 - (4 - (4 - ((4 - (4 - fluorophenyl) - 5, 6 - dihydropyridine -1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (40) (S)- 3 - (4 - (4 - ((4 - (4 - methoxyphenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (41) sodium (S)- 3 - (4 - (4 - ((3, 4 - dihydroquinoline -1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate; (42) potassium (S)- 3 - (4 - (4 - ((3, 4 - dihydroquinoline -1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate; (43) (S)- 3 - (4 - (4 - ((4 - (benzo thiazol - 2 - yl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (44) (S)- 3 - (4 - (4 - ((4 - (5 - propyl pyrimidine - 2 - yl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (45) (S)- 3 - (4 - (4 - ((4 - (5 - cyano - 2 - yl roh blood [tin[tin]) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (46) (3S) - 3 - (4 - (4 - ((3 - phenylpyrrolidin - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (47) sodium (S)- 3 - (4 - (4 - ((4 - (4 - methoxyphenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate; (48) (S)- 3 - (4 - (4 - (2 - (6 - methoxy - 3, 4 - dihydroisoquinoline -2 (1H) - yl) ethyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (49) (S)- 3 - (4 - (4 - (2 - (isoindolin - 2 - yl) ethyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (50) (S)- 3 - (4 - (4 - (2 - (3, 4 - dihydroisoquinoline -2 (1H) - yl) ethyl) benzyloxy) phenyl) - 4 - [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate wing; and (51) sodium (S)- 3 - (4 - (4 - ((6 - methoxy - 3, 4 - dihydroisoquinoline -2 (1H) - yl) methyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate. The compounds represented by formula 1 can be used in form of a pharmaceutically acceptable salt thereof, pharmaceutically acceptable salts include acid addition salts formed by useful in free acid (free acid). Acid addition salt hydrochloric acid, nitric acid, phosphoric acid, sulfuric acid, hydrobromic acid, iodine desalting dissipation, pH, inorganic species such as phosphorous, aliphatic mono and dicarboxylate, phenyl - substituted alkanoate, hydroxy alkanoate and alkanes d five A [thu[thu], aromatic lead-free, non-toxic organic acid such as aliphatic and aromatic the opinion phone it buys type, acetic acid, iodobenzoic acid, citric acid, lactic acid, maleic acid, a central, methanesulphonic acid, 4 - toluene sulfonic acid, tartaric acid, obtain from organic acid such as fumaric acid. This is efficient in salt types include sulfate, fatigue alkylsulfate, bisulphate, sulfite, sheet-snow, nitrate, phosphate, mono high draw [keyn[keyn] phosphate, dihydrogen phosphate, meta phosphate, pyrophosphate chloride, bromide, iodide, fluoride, acetate, propionate, car Roh A [thu[thu], relay [thu[thu] capric acid, acrylate, are fully capped, isobutyrate, capric acid rate, heptanoate, propy the [ley[ley] which comes the [thu[thu], oxalate, for the polymerization of olefins, three sour this [thu[thu], possibility hemp cloth [ley[ley] [thu[thu], three Kate, fumarate, maleate, father [thin[thin] radio - 1, 4 - hydroxycyclohexyl, hexane - 1, 6 - radio polycarbonate, benzoate, chloro benzoate, methyl benzoate, dinitro benzoate, hydroxybenzoate, the maul [thok[thok] city it cuts trillion A [thu[thu], phthalate, terephthalate, alkyl benzene sulfonate, toluene sulfonate, chlorobenzene sulfonate, xylene sulfonate, phenyl acetate, phenyl propionate, phenyl butyrate, citrate, lactate, β - hydroxybutyrate, glycolate, maleate, tart [ley[ley] [thu[thu], methane sulfonate, propane sulfonate, naphthalene - 1 - sulfonate, naphthalene - 2 - sulfonate, mandelate etc.. The acid addition salt can be prepared by conventional means, for example derivatives of formula 1 for use in a methanol, ethanol, acetone, methylene chloride, in an organic solvent such as acetonitrile melts the organic or inorganic acid by applying filtering the precipitate generated, increasing or, vacuum distilling a crude halogenated butyl rubber in excess of acid solvent wherein organic solvent can be crystallized. Also, pharmaceutically acceptable base can be made using metallic salts. For example alkali metal or alkaline earth metal salt of an alkali metal hydroxide or alkaline earth hydroxides or dissolved and excess of compound solution, filtering non-dissolved compound salt, filtrate evaporation, is dried to obtain. The, metal salt include sodium, potassium or calcium salts to produce pharmaceutically suitable. Also, alkali metal or alkaline earth metal salt corresponding salt by reacting suitable sound salt obtain (for example, the residual). Further, using a pharmaceutically acceptable organic acid bonded amino acid amino group can be salts, amino acid salts include for example, sterilization effects, alanine, phenylalanine, valine, lysine, the natural writing base hit [mik[mik] it buys pharmaceutically suitable for producing amino acids, most preferably L - lysine to produce pharmaceutically suitable. Also, the present invention relates to compounds of formula 1 and pharmaceutically acceptable salts as well as, can be therefrom solvates, optical isomers, such as hydrate comprising both. The pharmaceutical compositions of the present invention can be pharmaceutically acceptable active ingredient in addition to the recombinant. Pharmaceutical compositions of the invention comprising a pharmaceutically acceptable vehicle formulations typically when used as, lactose, dextrose, sucrose, sorbitol, mannitol, starch, acacia rubber, calcium phosphate, alginate, gelatin, calcium silicate, microcrystalline cellulose, polyvinylpyrrolidone, cellulose, water, syrup, methyl cellulose, methyl hydroxy benzoate, propyl hydroxy benzoate, talc, magnesium and mineral oils and the like acid including but, limited to are not correct. The pharmaceutical compositions of the present invention the components in addition to the lubricant, wetting agent, sweetener, flavoring agent, emulsifier, suspending agent, preservatives can be further comprises. Pharmaceutically acceptable formulations suitable carrier and Remington's Pharmaceutical Sciences (19th ed. , 1995) disclosed detailed. Also, effective doses of compounds of the present invention applicable to a patient's age, roller, sex, dosage forms, depending on the degree can be health conditions and diseases, generally about 0. 001 - 100 mg/kg/one and, preferably 0. 01 - 35 mg/kg/never. Roller based on the 70 kg patient is in adult, generally 0. 07 - 7000 mg/one and, preferably 0 direct wind. 7 - 2500 mg/one and, a physician or pharmacist may be administered at regular intervals 1 1 divided into many times to times only if disapproval. Oral or parenteral administration of the pharmaceutical compositions of the present invention can be, when parenteral administration, skin by topical application, intravenous injection, subcutaneous injection, muscle injection, abdominal implantation, such as transdermal administration can be administered. Preferably oral administration of the pharmaceutical compositions of the present invention can be, solid preparation for oral administration is purification, annularity system, inhaled, granules, capsule, such as included in one of the plurality of height system, such solid formulation contains at least one compounds of the invention at least one of one or more excipients for example, starch, calcium carbonate, sucrose (sucrose) or lactose (lactose) or gelatin pressure is lowered formulated as a substrate. Also, in addition to simple excipient lubricant such as talc [su[su] mote [ley[ley] [thu[thu] magnesium are also are used. Liquid formulations include suspending agent for oral administration, liquid content, corresponding to emulsion or syrup and the like, a simple diluent commonly used in water, in addition to the paraffin droplet various excipients, for example wetting agent, sweetener, fragrance, preservative can be like. For oral administration preparations contain a sterile aqueous solution, non-aqueous solvent, solvent suspension, emulsion, first frozen desiccant, like left system multiple myelomas are included. Non-aqueous solvent, suspension solvent include propylene glycol, polyethylene glycol, vegetable oil such as olive oil, an injectable the [ley[ley] which comes the [thu[thu] and ethyl ester is used as the alkali such as can be. left proposal base include above [theyp[theyp] brush (witepsol), mark with goal, twin (tween) 61, carcass five fingers, the it soaked, glycerol, gelatin or the like can be used. The pharmaceutical compositions of the present invention is provided with invention consultation can be users depending, pharmaceutically acceptable carrier and/or excipients formulate using prepared by unit dose amount is made in the form or can be prepared container of muffler. The solution dosage form oil or aqueous medium, be in the form of suspension or emulsion X first, second powder, granules, tablets or capsules may be in the form of a second, can be additionally contains a dispersant or stabilizer. In one embodiment of the invention, inhibitors of the present invention IV d [pheyp[pheyp] mote [til[til] mote day [cu[cu]at the time of hit writing [lip[lip] sequence is (Sitagliptin), billets it will bring near, [lip[lip] (Vildagliptin), [sak[sak] It buys the writing [lip[lip] (Saxagliptin), contamination in writing [lip[lip] (Linagliptin), reel four writing [lip[lip] (Teneligliptin), with egg writing [lip[lip] (Alogliptin), my Americawriting [lip[lip] (Gemigliptin), two toggles [lip[lip] (Dutogliptin), base (Berberine), loupe this year (Lupeol), selected from the group consisting of coffee (dandelion coffee) and red (red alder) [ayl[ayl] Compared toit is burnt it comes only either are disclosed. In one embodiment of the present invention, the sulfonylureas sequence is polyimide (Carbutamide) polymerization of carboxylic acid, butenoic [heyk[heyk] It buys America [tu[tu] (Acetohexamide), claw [lu[lu] pro group America [tu[tu] (Chlorpropamide), [thol[thol] Father hit America [tu[tu] (Tolbutamide), writing pizza id (Glipizide), gliclazide (Gliclazide), writing it cuts the lama id which will grow (Glibenclamide), writing step [lu[lu] world [tu[tu] (Glibornuride), writing [khwi[khwi] money (Gliquidone), writing small storms of life id (Glisoxepide), writing nose bleed America [tu[tu] (Glyclopyramide) and glimepiride (Glimepiride) selected from the group consisting either composed are disclosed. In one embodiment of the present invention, the tooth it will doze [tin[tin] D it comes sequence is (Rosiglitazone) rosiglitazone, pioglitazone (Pioglitazone), of preventing (Troglitazone), four toggle it burns the zone (Netoglitazone), rosiglitazone (Rivoglitazone) ribo, selected from the group consisting either hour writing it burns the zone (Ciglitazone) and rhodanine (Rhodanine) are disclosed. In one embodiment of the present invention, a bi-pharmaceutical sequence is metformin (Metformin), the pen gun it pushed (Phenformin), the vice-pawl it pushed (Buformin), pro it will not be nine (Proguanil), claw [lu[lu] pro nine will not be (Chlorproguanil), chlorhexidine (Chlorhexidine), polyamino profile pharmaceutical (Polyaminopropyl biguanide (PAPB)), selected from the group consisting poly [heyk[heyk] San'A idthe rack which will knowat the time of [tin[tin] (Alexidine) (Polihexanide) and either are disclosed. In one embodiment of the present invention, the emphasis SGLT2 inhibitor compounds of the present invention glycol ripple rosin (empagliflozin), selected from the group consisting (dapagliflozin) multi green onion writing ripple rosincar orwriting ripple rosin (canagliflozin) and either are disclosed. In one embodiment of the present invention, the blending of the substances of the invention 1 effective and effective 2 weight ratio 0. To 100:1 03:1 are disclosed. In another embodiment the mixing weight ratio 0. 03:1 to 30:1 and, in alternate embodiments the mixed in the weight ratio 0. To 10:1 03:1 are disclosed. Only, the composition of the invention is synthesized by the use, according to the aspect ratio by weight to 10 parts or a reduction in the signal controller specially mixed, special constraints mixing weight ratio is performed, and a second patient pathologies known properties of polarity 1 2 effective mixing and effective administration of a combination is more than adequate. In one embodiment of the present invention, the compositions of the invention to about GPR40 (G-a protein receptor 40) enzyme activity. GPR40 is G - protein-coupled receptor (GPCR) expressed mainly in insulin-secreting cells of the pancreas to, GPR40 expression profiles including various metabolic disorders to treatment of obesity and diabetes have potential utility. In the present invention, formula 1 1 first active ingredient compound, optical isomer, or a pharmaceutically acceptable salt thereof alone in the event of a GPR40 receptor activity rate evaluation result, at low concentrations in all embodiments according to the present invention compounds are 50% GPR40 receptor activation (EC50 ) Is identified to enhance its activating effect or an (example experiments 1 and 2, reference also 1) his car. Also, in the present invention, formula 1 1 first active ingredient compound, optical isomer, or a pharmaceutically acceptable salt drug metabolism CYP inhibition activity rate evaluation result, all embodiments according to the present invention CYP enzyme inhibitory activity when administered in combination with other drug compounds are low due to the effects of the administration of a combination concentration rate, when the administration of a combination for treating complications if any other medicaments as signal peptides (reference example 3 experiments). Further, in the present invention, formula 1 1 first active ingredient compound, optical isomer, or a pharmaceutically acceptable salt per load experiments result in oral, all embodiments according to the present invention in a reduction in the conventional known GPR40 active compounds are represented by the formula is as similar as shown is effective in vivo in good or excellent active GPR40 (experiment example 4, 5 and 6 reference) can be that of a significantly. Also, in the present invention, formula 1 1 first active ingredient compound, optical isomer, or a pharmaceutically acceptable salt concentration curve in experiments for evaluating blood oral administration of GLP-a 1 result, compared to a comparison example 1 compound is glucose treatment group (Veh.), but that is not effective blood concentration of GLP-a 1 appears to rise after administration, the embodiments in accordance with the 9 compounds when administered to SD for detecting BAX, increasing blood concentration of GLP-a 1 as signal peptides (experiment example 7, also reference 2). Further, according to the invention novel 3 - (4 - (benzyloxy) phenyl) - 4 - wing acid derivatives IV d [pheyp[pheyp] mote [til[til] mote day [cu[cu][heyk[heyk] inosinate (Dipeptidyl peptidase-a 4, DPPIV) inhibitors based, sulfonylureas (Sulfonylurea) sequence, tooth it will doze [tin[tin] D it comes (Thiazolidinediones, TZD) sequence, such as a representative drugs (Biguanide) series or SGLT2 inhibitor sequence-pharmaceutical rehmanniae, administration of the drugs alone better hypoglycemic effect as in the picomolar (for example 8 to 12 8 3 12 14 and also to table to reference the table of experiments also). That is, a kind of pharmaceutical compositions GPR40 cooled the hinges with active protein in insulin secretion is reduced, which in combination with other drug administration, is effective to activate protein in vivo GPR40 significantly up plots. In one embodiment of the present invention, the metabolic disease is obesity, diabetes of type I, diabetes type II, use my party, treating insulin resistance, hyperglycemia, aims, high the Holy Land bloodletting symptoms, hypercholesterolemia or mixed dyslipidemia, hypertriglyceridemia and X syndrome selected from the group consisting composers. When using the compositions of the present invention, represented by the formula described above by can be useful for prevention or treatment of metabolic disorders. Of producing compounds represented by the formula 1 of the present invention as follows: Preparation 1 According to the present invention is a compound represented by formula 1 1 as shown in the variation of producing compounds, compounds represented by formula 2 with a compound represented by formula 3 condensation reactions produce a compound represented by the formula 4 (step 1); and 1 formula 4 made in the steps of performing a reducing compound represented by the formula 1 compound for the production (step 2); connected to a cold air can. [Compound 1] (In the reactive 1, R1 , R2 , R3 , R4A , R4B , R5 , A, E, n and X comprises above are useful as defined in formula 1; The C Y1-10 A straight or branched alkyl are disclosed). Or less, according to the present invention represented by formula 1 of producing compounds processes detailed as follows. The method of producing compounds represented by formula 1, formula 2 with a compound represented by formula 3 above steps 1 compounds represented by compounds having coupling reactions represented by step represented by the formula 4, more specifically compounds of formula 2, tri-phenyl phosphine compounds of formula 3 with a solution mixing -5 °C to 10 °C (Mitsunobu reaction) by reaction at a temperature of azo carboxylates reagent america [chu[chu] outlived by performing slowly enemy producing compound formula 4 are disclosed. The, (diethyl azodicarboxylate, DEAD) ethyl azo die car [lu[lu] luck thread [ley[ley] [thu[thu] such azo carboxylates reagent include die, or die can be using isopropyl azo die car [lu[lu] luck thread [ley[ley] [thu[thu] (diisopropyl azodicarboxylate, DIAD), using a die-isopropyl azo die car [lu[lu] luck thread [ley[ley] [thu[thu] (diisopropyl azodicarboxylate, DIAD) can be. Also, the reaction solvent tetrahydrofuran mixable (THF), methane (DCM) to biocatalytic, toluene, etc. can be knight reel acetoacetoxy-functional, preferably tetrahydrofuran mixable (THF) can be using. Further, the reaction temperature is between 0 °C is preferably carried out in the boiling point of a solvent, the reaction time is special constraint cannot be, 0. 5 - 10 preferably reaction time. The method of producing compounds represented by formula 1, formula 4 1 2 the steps includes a step made in the compound represented by the formula 1 compounds having reduction reaction in the presence a base represented by performing step, more specifically the steps made in 1 formula 4 compound with a base at ambient temperature by reacting compounds of formula 4 is included in the formula 1 is represented by compounds having reduced with car luck practical technique ester are disclosed. The, potassium hydroxide (KOH) and the base include, sodium hydroxide (NaOH), lithium hydroxide (LiOH) can be etc., potassium hydroxide (KOH) can be preferably used. Also, the reaction solvent tetrahydrofuran mixable (THF), methane (DCM) to biocatalytic, toluene, etc. can be knight reel acetoacetoxy-functional, preferably tetrahydrofuran mixable (THF) can be using. Further, the reaction temperature is between 0 °C is preferably carried out in the boiling point of a solvent, the reaction time is special constraint cannot be, 0. 5 - 10 preferably reaction time. Starting material (compound formula 2) preparation According to the invention in the reactive 1, 2 with a defined compound a compound represented by chemical formula 2 as shown in the variation, With a compound represented by formula 8 9 10 reacting compounds represented by the general formula formula compound producing (step 1); The steps made in formula 1 compounds represented by the general formula compound formula 10 11 and 12 produce a reacting compound (step 2); and The steps 2 formula 12 made in a reducing compound represented by the formula 2 compound reactions producing (step 3); can be prepared by the method of manufacture which include. [Compound 2] (In the reactive 2, R1 , R2 , R3 , R4A , R4B , R5 , A, E, n and X is above are useful as defined in the formula 1, The triple base Oro methane opinion gun four sprouting, - OTf is are disclosed). Or less, according to the present invention represented by formula 2 of producing compounds processes specifically described as follows. According to the invention of producing compounds represented in formula 2, the steps 8 and 9 compound having a formula 1 compound having a formula represented by reacting compounds having formula 10 step, more specifically to compound comprises a monomer represented by the formula 8 compound to organic solvent dissolving the -70 °C -80 °C in formula 9, bis (tri for producing tris) amide metal complexes after slowly the enemy it inflicted, compounds represented by formula 10 can be added at ambient temperature to produce a temperature are disclosed. The, the bis (tri for producing tris) amide metal complexes include potassium bis (tri for producing tris) amide, lithium bis (tri for producing tris) amide, or sodium bis (tri for producing tris) using the amide can be, preferably potassium bis (tri for producing tris) the amide can be used. Also, the organic solvent (THF) tetrahydrofuran mixable, [thil[thil] Mote [lu[lu] die, die phenyl ether, isopropyl ether (DIPE) die, die methyl amide (DMF) formaldehyde, methyl acetamide (DMA), die methyl opinion bombing death id (DMSO), methane (DCM) to biocatalytic, chlorobenzene, toluene, benzene can be use. Further, the reaction temperature is preferably carried out in the boiling point of a solvent -80 °C between, the reaction time is special constraint cannot be, 0. 5 - 10 preferably reaction time. According to the invention of producing compounds represented in formula 2, compounds represented by formula 10 made in step 1 comprises the steps 2 compound represented by formula 11 and 12 step reacting compounds having the general formula, more specifically in the presence of palladium catalyst compounds represented by formula 10 made in the steps 1 and represented by formula 11 boronate (Suzuki coupling reaction) suzuki coupling reaction of compound represented by the formula 12 performing the production are disclosed. The, the tetrakis (tri-phenyl phosphine) palladium catalyst (Pd (PPh3 )4 ), Bis (tri-phenyl phosphine) palladium (II) die chloride (PdCl2 (PPh3 )2 ), Die palladium chloride (PdCl2 ), Palladium acetate (Pd (OCOCH3 )2 ) Can be etc., preferably tetrakis (tri-phenyl phosphine) (Pd (PPh3 )4 ) Can be used. Also, the organic solvent (THF) tetrahydrofuran mixable, die ethyl ether, die phenyl ether, isopropyl ether (DIPE) die, die methyl amide (DMF) formaldehyde, methyl acetamide (DMA), die methyl opinion bombing death id (DMSO), methane (DCM) to biocatalytic, chlorobenzene, toluene, benzene etc. can be, preferably toluene can be using. Further, the reaction temperature is between 0 °C is preferably carried out in the boiling point of a solvent, the reaction time is special constraint cannot be, 0. 5 - 10 preferably reaction time. According to the invention of producing compounds represented in formula 2, the steps 3 made in the step 2 in the presence of a compound represented by the formula 12 represented by step performing reduction reaction compounds having 2 represented by the formula, more specifically the steps 2 formula 12 made in an organic solvent dissolving the compound represented by formula 12 base is added hydroxy aldehyde compounds included in formula 2 produce a reduced compound are disclosed. The, the organic solvent methanol, ethanol, ethyl acetate, tetrahydrofuran mixable, die ethyl ether, or 2 using a mixed solution can be at least one, preferably tetrahydrofuran mixable: methanol (4:1) can be obtained by mixing a solution. Also, the base is an preferred embodiment sodium (NaBH3 ), Lithium id under aluminum id (LiAlH4 ) Can be etc., preferably sodium preferred embodiment (NaBH3 ) Can be used. Further, the reaction temperature is between 0 °C is preferably carried out in the boiling point of a solvent, the reaction time is special constraint cannot be, 0. 5 - 10 preferably reaction time. Preparation 2 According to the present invention is a compound represented by formula 1 3 as shown in the variation of producing compounds, compounds represented by formula 5 with a compound represented by formula 3 coupling reactions producing compounds represented by the formula 6 (step 1); The steps 1 through 6 made in the compounds represented by formula (Mesylate reaction) to produce a compound represented by the formula 7 mesylate reaction (step 2); The steps of mesylate (Mesylate) 2 formula 7 compound prepared in the compounds represented by formula 13 place substituting a compound represented by the formula 4 producing (step 3); and 3 formula 4 made in the steps of performing a reducing compound represented by the formula 1 compound for the production (step 4); can be prepared by the method of manufacture which include. [Compound 3] (In the reactive 3, R1 , R2 , R3 , R4A , R4B , R5 , A, E, n and X comprises above are useful as defined in formula 1; The C Y1-10 A straight or branched alkyl are disclosed). Or less, according to the present invention represented by formula 1 of producing compounds processes detailed as follows. The method of producing compounds represented by formula 1, formula 1 compounds represented by formula 5 with a compound 3 represented by the steps of performing the coupling of producing compounds represented by the formula 6 are disclosed. The, the organic solvent (THF) tetrahydrofuran mixable, die ethyl ether, die phenyl ether, isopropyl ether (DIPE) die, die methyl amide (DMF) formaldehyde, methyl acetamide (DMA), die methyl opinion bombing death id (DMSO), methane (DCM) to biocatalytic, chlorobenzene, toluene, benzene etc. can be, preferably methyl amide (DMF) can be using formaldehyde. Also, the base is an and the base is an cesium carbonate (Cs2 CO3 ), Sodium preferred embodiment (NaBH3 ), Lithium id under aluminum id (LiAlH4 ) Can be etc., preferably cesium carbonate (Cs2 CO3 ) Can be used. Further, the reaction temperature is between 0 °C is preferably carried out in the boiling point of a solvent, the reaction time is special constraint cannot be, 0. 5 - 10 preferably reaction time. According to the invention of producing compounds represented in formula 1, the steps includes a step 1 2 6 made in the compounds represented by formula (Mesylate reaction) compound represented by the formula 7 in mesylate reaction solvent through the production are disclosed. The, the mesylate used for the sample using methane methylsulfonyl chloride (Methanesulfonyl chloride, MsCl) can be. Also, triethylamine (TEA) the organic solvent, tetrahydrofuran mixable (THF), die ethyl ether, die phenyl ether, isopropyl ether (DIPE) die, die methyl amide (DMF) formaldehyde, methyl acetamide (DMA), die methyl opinion bombing death id (DMSO), methane (DCM) to biocatalytic, chlorobenzene, toluene, benzene etc. can be, preferably using triethylamine (TEA) can be. Further, the reaction temperature is between 0 °C is preferably carried out in the boiling point of a solvent, the reaction time is special constraint cannot be, 0. 5 - 10 preferably reaction time. According to the invention of producing compounds represented in formula 1, the compound of formula 7 3 the steps made in step 2 (Mesylate) compounds represented by formula 13 substituting a mesylate compound represented by the formula 4 place producing are disclosed. The, the organic solvent (THF) tetrahydrofuran mixable, die ethyl ether, die phenyl ether, isopropyl ether (DIPE) die, die methyl amide (DMF) formaldehyde, methyl acetamide (DMA), die methyl opinion bombing death id (DMSO), methane (DCM) to biocatalytic, chlorobenzene, toluene, benzene etc. can be, preferably using biocatalytic methane (DCM) can be. Also, the base is an and the base is an cesium carbonate (Cs2 CO3 ), Sodium preferred embodiment (NaBH3 ), Lithium id under aluminum id (LiAlH4 ) Can be etc., preferably cesium carbonate (Cs2 CO3 ) Can be used. Further, the reaction temperature is between 0 °C is preferably carried out in the boiling point of a solvent, the reaction time is special constraint cannot be, 0. 5 - 10 preferably reaction time. The method of producing compounds represented by formula 1, formula 4 made in step 3 comprises the steps 4 compound represented by the formula 1 compounds having reduction reaction in the presence a base represented by performing step, more specifically the steps made in 3 formula 4 compound with a base at ambient temperature by reacting compounds of formula 4 is included in the formula 1 is represented by compounds having reduced with car luck practical technique ester are disclosed. The, potassium hydroxide (KOH) and the base include, sodium hydroxide (NaOH), lithium hydroxide (LiOH) can be etc., potassium hydroxide (KOH) can be preferably used. Also, the reaction solvent tetrahydrofuran mixable (THF), methane (DCM) to biocatalytic, toluene, etc. can be knight reel acetoacetoxy-functional, preferably tetrahydrofuran mixable (THF) can be using. Further, the reaction temperature is between 0 °C is preferably carried out in the boiling point of a solvent, the reaction time is special constraint cannot be, 0. 5 - 10 preferably reaction time. Preparation 3 According to the invention of producing compounds represented by formula 1 is a compound 4 as shown in the variation, represented by the formula 1a compound obtained by ring-opening reactions produce a compound represented by the formula 1b (step 1); connected to a cold air can. [Compound 4] (4 in the reactive, R1 The above are useful as defined in formula 1; The compound represented by formula 1 compounds formula 1a and 1b multiple myelomas are included). Or less, the manufacturing method according to the invention detailed processes as follows. In the manufacturing method according to the invention, the steps of performing an electrochemical reaction in the presence of a formula 1 compound represented by the formula 1b 1a acid compounds having step represented by ring opening, more specifically the formula 1 compound represented by chemical formula 1a acid compound in the presence of n-heterocyclic compounds of formula 1a by ring opening reaction by producing the dienophile compound of formula 1b is obtained by ring-opening comprising carbonate are disclosed. The, the acid hydrochloric, sulfuric acid, phosphoric acid can be used such as inorganic acid, preferably hydrochloric acid can be used. Also, the reaction solvent tetrahydrofuran mixable (THF), methane (DCM) to biocatalytic, toluene, etc. can be knight reel acetoacetoxy-functional, preferably tetrahydrofuran mixable (THF) can be using. Further, the reaction temperature is between 0 °C is preferably carried out in the boiling point of a solvent, the reaction time is special constraint cannot be, 0. 5 - 10 preferably reaction time. Preparation 4 According to the invention of producing compounds represented by formula 1 is a compound 5 as shown in the, compound represented by the formula 1c formula 1b reactions produce a reducing compound (step 1); connected to a cold air can. [Compound 5] (In the reactive 5, R1 The above are useful as defined in formula 1; The compound represented by formula 1 compounds formula 1b and 1c multiple myelomas are included). Or less, the manufacturing method according to the invention detailed processes as follows. In the manufacturing method according to the invention, the steps of performing an electrochemical reaction in the presence of a compound represented by formula 1b 1 represented by the formula 1c compounds having reduction represented by step, more specifically the compound formula 1 compound formula 1b one base formula 1b by reduction reaction in the presence of hydroxy carbonate compound represented by the formula 1c neel giga compounds having reduced are disclosed. The, preferred embodiment the base is an sodium (NaBH3 ), Lithium id under aluminum id (LiAlH4 ) Can be etc., preferably sodium preferred embodiment (NaBH3 ) Can be used. Also, the reaction solvent tetrahydrofuran mixable (THF), methane (DCM) to biocatalytic, toluene, etc. can be knight reel acetoacetoxy-functional, preferably tetrahydrofuran mixable (THF) can be using. Further, the reaction temperature is between 0 °C is preferably carried out in the boiling point of a solvent, the reaction time is special constraint cannot be, 0. 5 - 10 preferably reaction time. Also, the present invention relates to compounds of formula 1, their isomers, or a pharmacologically acceptable salt as active ingredients containing pharmaceutical compositions for preventing or treating metabolic disorders. The, the pharmaceutical compositions unexpectedly give rise GPR40 activating enzymes characterized. GPR40 is G - protein-coupled receptor (GPCR) expressed mainly in insulin-secreting cells of the pancreas to, GPR40 expression profiles including various metabolic disorders to treatment of obesity and diabetes have potential utility. In this regard the compound formula 1, their isomers, or pharmaceutically acceptable salts GPR40 receptor activity rate evaluation result, at low concentrations in all embodiments according to the present invention compounds are 50% CPR40 receptor activation (EC50 ) Is identified to enhance its activating effect or an (example experiments 1 and 2, reference also 1) his car. Also, the compounds of formula 1, their isomers, or a pharmaceutically acceptable salt drug metabolism CYP inhibition activity rate evaluation result, all embodiments according to the present invention CYP enzyme inhibitory activity when administered in combination with other drug compounds are low due to the effects of the administration of a combination concentration rate, when the administration of a combination for treating complications if any other medicaments as signal peptides (reference example 3 experiments). Further, the compounds of formula 1, their isomers, or pharmaceutically acceptable salts result in load experiments per oral, all embodiments according to the present invention in a reduction in the conventional known GPR40 active compounds are represented by the formula is as similar as shown is effective in vivo in good or excellent active GPR40 (experiment example 4, 5 and 6 reference) can be that of a significantly. Also, the compounds of formula 1, their isomers, or pharmaceutically acceptable salts concentration curve in experiments for evaluating blood oral administration of GLP-a 1 result, compared to a comparison example 1 compound is glucose treatment group (Veh.), but that is not effective blood concentration of GLP-a 1 appears to rise after administration, the embodiments in accordance with the 9 compounds when administered to SD for detecting BAX, increasing blood concentration of GLP-a 1 as signal peptides (experiment example 7, also reference 2). Further, according to the invention novel 3 - (4 - (benzyloxy) phenyl) - 4 - wing acid derivatives IV d [pheyp[pheyp] mote [til[til] mote day [cu[cu][heyk[heyk] inosinate (Dipeptidyl peptidase-a 4, DPPIV) inhibitors based, sulfonylureas (Sulfonylurea) sequence, tooth it will doze [tin[tin] D it comes (Thiazolidinediones, TZD) sequence, or bi-pharmaceutical representative drugs (Biguanide) family of rehmanniae, administration of the drugs alone better hypoglycemic effect as in the picomolar (experiment example 8, 9, 10 and 11 of table 8, table 9, table 10, table 11, also 3, also 4, 5 and 6 may also reference). Thus, the compounds represented by formula 1 in insulin secretion is reduced if the cooled GPR40 active protein, which in combination with other drug administration, in vivo GPR40 activating protein is significantly and low heat curable compositions containing effective obesity, diabetes of type I, diabetes type II, coincidental glucagon-like peptide, insulin resistance, hyperglycemia, aims to, high the Holy Land bloodletting symptoms, hypercholesterolemia or mixed dyslipidemia, hypertriglyceridemia, such as sick X can be useful pharmaceutical compositions for preventing or treating metabolic disorders. The compounds of formula 1 can be administered oral and parenteral various formulations clinician upon administration, there is usually formulated using filler, extender, binder, wetting agent, disintegrating agent, surfactant manufactured by using the colorant such as diluent or excipient. Purification is a solid preparation for oral administration, annularity system, inhaled, granules, capsule, such as included in one of the plurality of height system, such solid formulation contains at least one compounds of the invention at least one of one or more excipients for example, starch, calcium carbonate, sucrose (sucrose) or lactose (lactose) or gelatin pressure is lowered formulated as a substrate. Also, in addition to simple excipient lubricant such as talc [su[su] mote [ley[ley] [thu[thu] magnesium are also are used. Liquid formulations include suspending agent for oral administration, liquid content, corresponding to emulsion or syrup and the like, commonly used for producing a simple diluent, in addition to the paraffin droplet various excipients, for example wetting agent, sweetener, fragrance, preservative can be like. For parental administration preparations contain a sterile aqueous solution, non-aqueous solvent, solvent suspension, emulsion, freeze-dried preparation, like left system multiple myelomas are included. Non-aqueous solvent, suspension solvent include propylene glycol, polyethylene glycol, vegetable oil such as olive oil, an injectable the [ley[ley] which comes the [thu[thu] and ethyl ester is used as the alkali such as can be. Base include above [theyp[theyp] brushleft proposal (witepsol), mark with goal, twin (tween) 61, carcass five fingers, the it soaked, glycerol, gelatin or the like can be used. Also, effective doses of compounds of the present invention applicable to a patient's age, roller, sex, dosage forms, depending on the degree can be health conditions and diseases, generally about 0. 001 - 100 mg/kg/one and, preferably 0. 01 - 35 mg/kg/never. Roller based on the 70 kg patient is in adult, generally 0. 07 - 7000 mg/one and, preferably 0. 7 - 2500 mg/one and, a physician or pharmacist may be administered at regular intervals 1 1 divided into many times to times only if disapproval. Or less, and positive examples for practicing the invention process by the experiments detailed as follows. Stage, exemplified embodiments of the invention to embodiments and experiments only, limited to the contents of the present invention are not correct. <Manufacturing example 1>ethyl 3 - (4 -Hydroxyphenyl)[heyk[heyk]- 4 -Polycarbonate of inosinate Manufacturing Nitrogen atmosphere, 250 ml cap to 3 - (4 - hydroxyphenyl) - 4 - tooth [heyk[heyk] - inosinate (20. 0 g) and ethanol (200 ml) a portion of the agitating after dissolving, sulfuric acid at room temperature (9. 6 ml) slowly enemy a-gate. 6 hours then reflux stirring the reaction terminates into distilled water (150 ml) is dripped evaporants then ethyl acetate (200 ml) extracted from the injection. The extracted organic layer reduced pressure drying target compound (19. 5 g, 85. 7%) by Congress. 1 H NMR (400MHz, CDCl3 ): Δ 7. 25 (2H, d), 6. 78 (2H, d), 4. 95 (1H, s), 4. 14 (2H, m), 4. 04 (1H, m), 2. 68 (2H, m), 1. 84 (3H, d), 1. 29 (3H, t). <Manufacturing example 2>(S)- ethyl 3 - (4 -Hydroxyphenyl)[heyk[heyk]- 4 -Polycarbonate of inosinate Manufacturing Nitrogen atmosphere, (S) 250 ml cap to - 3 - (4 - hydroxyphenyl) - 4 - tooth [heyk[heyk] - inosinate (20. 0 g) and ethanol (200 ml) a portion of the agitating after dissolving, sulfuric acid at room temperature (9. 6 ml) slowly enemy a-gate. 6 hours then reflux stirring the reaction terminates into distilled water (150 ml) is dripped evaporants then ethyl acetate (200 ml) extracted from the using. The extracted organic layer reduced pressure drying target compound (21. 2 g, 93. 2%) by Congress. 1 H NMR (400MHz, CDCl3 ): Δ 7. 25 (2H, d), 6. 78 (2H, d), 4. 95 (1H, s), 4. 14 (2H, m), 4. 04 (1H, m), 2. 68 (2H, m), 1. 84 (3H, d), 1. 29 (3H, t). <Manufacturing example 3>(R)- ethyl 3 - (4 -Hydroxyphenyl)[heyk[heyk]- 4 -Polycarbonate of inosinate Manufacturing Nitrogen atmosphere, (R)- 3 - the cap 250 ml (4 - hydroxyphenyl) - 4 - tooth [heyk[heyk] - inosinate (20. 0 g) and ethanol (200 ml) a portion of the agitating after dissolving, sulfuric acid at room temperature (9. 6 ml) slowly enemy a-gate. 6 hours then reflux stirring the reaction terminates into distilled water (150 ml) is dripped evaporants then ethyl acetate (200 ml) extracted from the using. The extracted organic layer reduced pressure drying target compound (20. 6 g, 90. 6%) by Congress. 1 H NMR (400MHz, CDCl3 ): Δ 7. 25 (2H, d), 6. 78 (2H, d), 4. 95 (1H, s), 4. 14 (2H, m), 4. 04 (1H, m), 2. 68 (2H, m), 1. 84 (3H, d), 1. 29 (3H, t). <Manufacturing example 4>(3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)Phenyl) Of methanolbath Step 1:1,4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - one Coating fluoromethane sulfonylBy means of a Nitrogen atmosphere, 1000 ml cap to 1. 4 - oxa cyber to die [4. 5] orl - 8 - on (30. 0 g) and toluene (300 ml) which can be after dissolving agitating, N - phenyl bis (tri [phul[phul] Base Oro methane opinion phone already the [tu[tu] (64. 3 g) was added. Then, in -78 °C 0. 7M solution of potassium bis (tri for producing tris) amide (257 ml) stirring at ambient temperature for 4 hours using a dropping funnel and wherein the temperature slowly enemy slowly-gate. The reaction terminates distilled water (200 ml) enemy evaporants and ethyl acetate (300 ml) are extracted using, extracted organic layer reduced pressure drying target compound (54. 7 g, 98. 8%) by Congress. 1 H NMR (400MHz, CDCl3 ): Δ 5. 68 (1H, t), 4. 01 (4H, s), 2. 55 (2H, t), 2. 42 (2H, d), 1. 92 (2H, t). Step 2:3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)Of benzaldehyde firstbath Nitrogen atmosphere, 1000 ml cap to 1. 4 - oxa cyber to die [4. 5] - 7 - en - 8 - yl trifluoromethanesulfonate a process (54. 70 g) and toluene (300 ml) which can be after dissolving agitating, 3 - gun closing Neel step theory it buys (28. 7 g) purification and cesium (156 g) was added. Then, the tetrakis (tri-phenyl phosphine) palladium 0 °C cooling (11. 09 g) stirring at ambient temperature for 3 hours while the temperature slowly adding a back-gate. The reaction terminates distilled water (200 ml) enemy evaporants and ethyl acetate (300 ml) are extracted using, extracted organic layer reduced pressure drying target compound (45. 9 g, 99%) was obtained. 1 H NMR (400MHz, CDCl3 ): Δ 10. 03 (1H, s), 7. 92 (1H, s), 7. 76 (1H, d), 7. 67 (1H, d), 7. 47 (1H, t), 6. 11 (1H, s), 4. 05 (4H, s), 2. 71 (2H, t), 2. 51 (2H, s), 1. 97 (2H, t). Step 3: (3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)Phenyl) Of methanol Nitrogen atmosphere, 500 ml cap to 3 - (1. 4 - oxa cyber to die [4. 5] - 7 - en - 8 - process for one) benzaldehyde (46. 9 g), tetrahydrofuran mixable (160 ml) and methanol (40 ml) which can be after dissolving agitating, 0 °C been cooling. Then, sodium at step low id id (10. 9 g) stirring at ambient temperature for 3 hours by adding evaporants temperature-gate. The reaction terminates distilled water (150 ml) and enemy evaporants ethyl acetate (150 ml) are extracted using, extracted organic layer reduced pressure drying target compound (37. 8 g, 81. 7%) by Congress. 1 H NMR (400MHz, CDCl3 ): Δ 7. 34 (1H, s), 7. 25 (3H, m), 6. 01 (1H, m), 4. 69 (2H, d), 4. 04 (4H, s), 2. 68 (2H, m), 2. 48 (2H, s), 1. 94 (2H, t), 1. 80 (1H, t). <Manufacturing example 5>(4 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)Phenyl) Of methanolbath Step 1:4 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)Of benzaldehyde firstbath Nitrogen atmosphere, 250 ml cap to 1. 4 - oxa cyber to die [4. 5] - 7 - en - 8 - yl trifluoromethanesulfonate a process (3. 0 g) and toluene (50 ml) which can be after dissolving agitating, 3 - formyl phenyl boronic acid (1. 8 g) purification and cesium (8. 47 g) is added, with respect to the cooled to 0 °C. Then, tetrakis (tri-phenyl phosphine) palladium (601 mg) slowly adding a high temperatures-gate 3 hours by stirring. The reaction terminates distilled water (500 ml) enemy evaporants and ethyl acetate (100 ml) are extracted using, extracted organic layer reduced pressure drying target compounds (2. 0 g, 78. 7%) by Congress. 1 H NMR (400MHz, CDCl3 ): Δ 10. 00 (1H, s), 7. 84 (2H, d), 7. 57 (2H, d), 6. 19 (1H, s), 4. 06 (4H, s), 2. 71 (2H, t), 2. 53 (2H, s), 1. 97 (2H, t). Step 2: (4 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)Phenyl) Of methanol Nitrogen atmosphere, 250 ml cap to 4 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzaldehyde (2. 0 g), tetrahydrofuran mixable (40 ml) and methanol (10 ml) which can be after dissolving agitating, 0 °C been cooling. Then, sodium at step low id id (619 mg) at ambient temperature for 3 hours by adding evaporants temperature stirring-gate. The reaction terminates distilled water (50 ml) and enemy evaporants ethyl acetate (100 ml) are extracted using, extracted organic layer reduced pressure drying target compound (1. 6 g, 52. 9%) by Congress. 1 H NMR (400MHz, CDCl3 ): Δ 7. 40 (2H, d), 7. 32 (2H, d), 6. 01 (1H, m), 4. 70 (2H, d), 4. 13 (4H, s), 2. 68 (2H, t), 2. 49 (2H, s), 1. 93 (2H, t), 1. 60 (1H, t). <Manufacturing example 6>ethyl 3 - (4 - (4 - ((Methyl opinion gun Neel jade city)Methyl)Benzyloxy)Phenyl)[heyk[heyk]- 4 -InosinatePolycarbonate Step 1: (4 - (Bromo methyl)Phenyl) Of methanol Nitrogen atmosphere, methyl 4 - (bromo methyl) benzoate 5 to cap 1L. 0g and agitating a portion of the MC 20 ml after dissolving, in a slowly enemy -78 °C DIBAL-a H 70 ml-gate. 5 time been rapidly, then slowly lowers the reaction terminates into a 0 °C using MC the enemy it inflicted extracted from the chuck. The extracted organic layer reduced pressure drying desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 42 (2H, d), 7. 38 (2H, d), 4. 73 (2H, s), 4. 52 (2H, m). Step 2: ethyl 3 - (4 - (4 - (Hydroxymethyl)Benzyloxy)Phenyl)[heyk[heyk]- 4 -Polycarbonate of inosinateManufacturing Nitrogen atmosphere, obtained in the manufacturing example 1 ethyl 3 - 500 ml flasks (4 - hydroxyphenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate 4. 0g obtained in the step 1 (4 - (bromo methyl) phenyl) methanol 5. 0g dissolving Cs agitating a DMF 50 ml is placed2 CO3 9. 0g the enemy it inflicted 12 after a time-gate stirring at room temperature. By extracting the reaction terminates after washing the ethyl acetate then slowly the enemy it inflicted chuck working phosphorus, magnesium sulfate anhydride and concentrated drying the substrate. Then, silica column chromatography separation desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 42 (2H, d), 7. 38 (2H, d), 7. 29 (2H, d), 6. 93 (2H, d), 5. 06 (2H, s), 4. 73 (2H, d), 4. 15 (2H, m), 4. 06 (1H, m), 2. 68 (2H, m), 1. 84 (3H, s), 1. 69 (1H, m), 1. 24 (3H, m). Step 3: ethyl 3 - (4 - (4 - ((Methyl opinion gun Neel jade city)Methyl)Benzyloxy)Phenyl) [heyk[heyk]- 4 -InosinatePreparation of polycarbonate Nitrogen atmosphere, obtained in step 3 - 2 the cap 500 ml ethyl (4 - (4 - (hydroxymethyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate 3. 0g dissolving TEA 4 MC 30 ml agitating a sugar syrup. In a enemy 0 ml 0 °C-gate. 30 minutes, MsCl 2. A 1 ml enemy and slowly, slowly the enemy it inflicted MC 1 reaction time using the extracted from the chuck then terminates. The extracted organic layer reduced pressure drying desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 49 (4H, m), 7. 29 (2H, d), 6. 93 (2H, d), 5. 27 (2H, s), 5. 08 (2H, s), 4. 15 (2H, m), 4. 06 (1H, m), 2. 95 (3H, s), 2. 68 (2H, m), 1. 84 (3H, s), 1. 69 (1H, m), 1. 24 (3H, m). <Manufacturing example 7>(S)- ethyl 3 - (4 - (4 - ((Methyl opinion gun Neel jade city)Methyl)Benzyloxy)Phenyl)[heyk[heyk]- 4 -Polycarbonate of inosinate Step 1: (S)- ethyl 3 - (4 - (4 - (Hydroxymethyl)Benzyloxy)Phenyl)[heyk[heyk]- 4 -Inosinate methodProduced Ethyl 3 - (4 - hydroxyphenyl) - 4 - inosinate [heyk[heyk] formation employed in place, (S)- ethyl 3 - (4 - hydroxyphenyl) - 4 - but inosinate [heyk[heyk] formation using, for example 6 step 2 performs in a manner that said the same desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 42 (2H, d), 7. 38 (2H, d), 7. 29 (2H, d), 6. 93 (2H, d), 5. 06 (2H, s), 4. 73 (2H, d), 4. 15 (2H, m), 4. 06 (1H, m), 2. 68 (2H, m), 1. 84 (3H, s), 1. 69 (1H, m), 1. 24 (3H, m). Step 2: (S)- ethyl 3 - (4 - (4 - ((Methyl opinion gun Neel jade city)Methyl)Benzyloxy)Phenyl)[heyk[heyk]- 4 -theroh A [thu[thu] manufacturing Ethyl 3 - (4 - (4 - (hydroxymethyl) benzyloxy) phenyl) - 4 - formation [heyk[heyk] inosinate instead, 3 - ethyl - 1 obtained in the steps (S) (4 - (4 - (hydroxymethyl) benzyl) phenyl) - 4 - inosinate [heyk[heyk] formation but using, performs in a manner that said desired compound example 3 step equal to 6 are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 49 (4H, m), 7. 29 (2H, d), 6. 93 (2H, d), 5. 27 (2H, s), 5. 08 (2H, s), 4. 15 (2H, m), 4. 06 (1H, m), 2. 95 (3H, s), 2. 68 (2H, m), 1. 84 (3H, s), 1. 69 (1H, m), 1. 24 (3H, m). <Manufacturing example 8>6 -Methoxy- 1, 2, 3, 4 -Of tetrahydro isoquinoline Manufacturing Step 1:3 - ethylmaul [thok[thok] hour lung four [thil[thil] car May [thu[thu] Manufacturing Nitrogen atmosphere, cap to 2 - (3 - methoxyphenyl) ethanamine 25g is placed MC 300 ml agitating a dissolving, TEA 24. In a 2 ml enemy 0 °C-gate. 30 minutes, ethyl chloroformate 16. A 6 ml enemy and slowly, slowly enemy MC and using the reaction terminates after some time 1 extracted from the chuck. The extracted organic layer reduced pressure drying desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 25 (1H, m), 6. 79 (3H, m), 4. 70 (1H, s), 4. 13 (2H, m), 3. 81 (3H, s), 3. 46 (2H, m), 2. 80 (2H, m), 1. 25 (3H, m). Step 2:6 -Methoxy- 3, 4 -Dihydro isoquinoline-1 (2H) - wastewater treatment Nitrogen atmosphere, 500 ml ethyl 3 - 1 obtained in the step a poly gun [su[su] gun reel [lik[lik]maul [thok[thok] hour lung four [thil[thil] car May [thu[thu] 36g [eyk[eyk] Hour [tu[tu] 120g and agitating flasks dissolving 3 hours heating reflux was. After temperature is decreased down to the normal temperature, ethyl acetate is slowly extracted from the chuck enemy 3 or more times. After washing the organic layer extracted working phosphorus, magnesium sulfate anhydride was dried and concentrated. Then, silica column chromatography separation desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 8. 03 (1H, d), 6. 87 (1H, d), 6. 72 (1H, s), 6. 44 (1H, s), 3. 86 (3H, s), 3. 57 (2H, m), 2. 98 (2H, m). Step 3:6 -Methoxy- 1, 2, 3, 4 -Of tetrahydro isoquinoline Manufacturing Nitrogen atmosphere, 6 - methoxy - 3, 4 - dihydro isoquinoline -1 obtained in the step 2 flasks (2H) - dissolving in a sugar syrup 0 °C on 10g THF 150 ml agitating LAH 4. 3g enemy a slowly-gate. 5 hours refluxing ethyl acetate then slowly heating the reaction terminates after washing the phosphorus to chuck the working the enemy it inflicted extraction, anhydrous magnesium sulfate and concentrated drying out. Then, solidified desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 6. 94 (1H, d), 6. 73 (1H, d), 6. 65 (1H, s), 4. 14 (2H, s), 3. 80 (3H, s), 3. 13 (2H, m), 2. 79 (2H, m). <Manufacturing example 9>4 - (4 - (Methylsulfonyl)Phenyl) - 1, 2, 3, 6 -Tetrahydro pyridineHydrogelChloride manufacturing Step 1: [thu[thu]Butyl - 4 - (4 - (Methylsulfonyl)Phenyl) - 5, 6 -Dihydropyridine-1 (2H) -carTri-acrylate Nitrogen atmosphere, 4 - butyl - 5, 6 - dihydropyridine -1 [thu[thu] to cap 1000 ml (triple base Oro methyl opinion gun Neel jade city) - (2H) - car diplopia [ley[ley] [thu[thu] 3. 31g and toluene 50 ml agitating which can be after dissolving, 4 - (methylsulfonyl) phenyl at step Nick[eyk[eyk] Hour [tu[tu] 2. 0g and cesium purification of 6. 6g some excellent. Then, the tetrakis (tri-phenyl phosphine) palladium 0 °C cooling 1. 16g stirring at ambient temperature for 3 hours while the temperature slowly adding a back-gate. The reaction terminates after extracted using ethyl acetate and slowly enemy chuck, dry silica column extracted organic layer after separating desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 92 (2H. D), 7. 56 (2H, d), 6. 21 (1H, s), 4. 14 (2H, d), 3. 68 (2H, m), 3. 07 (3H, s), 2. 56 (2H, s), 1. 49 (9H, s). Step 2:4 - (4 - (Methylsulfonyl)Phenyl) - 1, 2, 3, 6 -Tetrahydro pyridineReceptacle hydrogelWindow of synthetic leather The steps 1 - butyl [thu[thu] obtained in 4 - (4 - (methylsulfonyl) phenyl) - 5, 6 - dihydropyridine - 1 (2H) -1 car diplopia [ley[ley] [thu[thu]. 4g after melting the MC 20 ml 4N HCl 10. 4 ml enemy a-gate. 5 hours, the reaction terminates after diethyl ethers the enemy it inflicted solidified desired compound are obtained. 1 H NMR (400MHz, D2 O): δ 7. 92 (2H. D), 7. 56 (2H, d), 6. 21 (1H, s), 4. 14 (2H, d), 3. 68 (2H, m), 3. 07 (3H, s), 2. 56 (2H, s). <Manufacturing example 10>4 - (1, 2, 3, 6 -Tetrahydro pyridine- 4 - yl) phenol claw hydrogelFor its production Step 1: [thu[thu]Butyl - 4 - (4 -Hydroxyphenyl) - 5, 6 -Dihydropyridine-1 (2H) -For granulatinghour [ley[ley] [thu[thu] manufacturing 4 - (methylsulfonyl) phenyl at step Nick[eyk[eyk] Hour [tu[tu] instead, but using 4 - hydroxy at hour phenyl step Nick[eyk[eyk] Hour [tu[tu], example 9 step 1 performs in a manner equal to said desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 26 (2H, d), 6. 83 (2H, d), 5. 93 (1H, s), 5. 47 (1H, s), 4. 07 (2H, s), 3. 66 (2H, m), 2. 50 (2H, s), 1. 52 (9H, s). Step 2:4 - (1, 2, 3, 6 -Tetrahydro pyridine- 4 - yl) phenol Of hydrochlorideManufacturing [thu[thu] - butyl 4 - (4 - (methylsulfonyl) phenyl) - 5, 6 - dihydropyridine -1 car diplopia [ley[ley] [thu[thu] (2H) - instead, the steps 1 - butyl [thu[thu] obtained in 4 - (4 - hydroxyphenyl) - (2H) - 5, 6 - dihydropyridine -1 car diplopia [ley[ley] [thu[thu] but using, example 9 step 2 performs in a manner that said the same desired compound are obtained. 1 H NMR (400MHz, D2 O): δ 7. 26 (2H, d), 6. 83 (2H, d), 5. 93 (1H, s), 5. 47 (1H, s), 4. 07 (2H, s), 3. 66 (2H, m), 2. 50 (2H, s). <Manufacturing example 11>4 - (4 - (3 - (Methylsulfonyl)Propoxy)Phenyl) - 1, 2, 3, 6 -2 high draw bloodPreparation of pyrrolidine hydrochloride Step 1:3 - (Methyl be enveloped) Propyl 4 -Methylbenzene alkylbenzene sulfonates Manufacturing Nitrogen atmosphere, 3 - propane - 1 - ol 25 to cap 500 ml (methyl be enveloped). 4g MC 500 ml TEA 44 ml 0 °C-gate in a dissolving enemy agitating a sugar syrup. 30 minutes, the reaction terminates after some time after the chuck 1 by slowly enemy TsCl 46g the enemy it inflicted slowly, using extracted from the MC. The extracted organic layer reduced pressure drying desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 81 (2H, d), 7. 38 (2H, d), 4. 16 (2H, m), 2. 53 (2H, m), 2. 47 (3H, s), 2. 05 (3H, s), 1. 94 (2H, m). Step 2:3 - (Methylsulfonyl) Propyl 4 -Methylbenzene alkylbenzene sulfonates Manufacturing Nitrogen atmosphere, 4 - methylbenzene sulfonate (methyl be enveloped) obtained in the step 3 - 1 profile a 62g flasks THF/150/100 ml distilled water in a dissolving (oxone) 0 °C enemy 310g is placed agitating you oxo-gate. After stirring at room temperature 12 time, then ethyl acetate the enemy it inflicted evaporants distilled water by extracting the reaction terminates after washing the working phosphorus, magnesium sulfate anhydride and concentrated drying desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 81 (2H, d), 7. 38 (2H, d), 4. 20 (2H, m), 3. 13 (2H, m), 2. 93 (3H, s), 2. 48 (3H, s), 2. 23 (2H, m). Step 3: [thu[thu]Butyl - 4 - (4 - (3 - (Methylsulfonyl)Propoxy)Phenyl) - 5, 6 -DihydroPyridine -1 (2H) - car diplopia [ley[ley] [thu[thu] manufacturing Example 10 1 - butyl 4 - [thu[thu] obtained in said step (4 - hydroxyphenyl) - 3 - (methylsulfonyl) - 5, 6 - dihydropyridine -1 car diplopia [ley[ley] [thu[thu] and (2H) 4 - methylbenzene sulfonate obtained in the above steps 2 using profile but, example 6 step 2 performs in a manner that said the same desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 34 (2H, d), 6. 85 (2H, d), 6. 00 (1H, s), 4. 12 (2H, s), 3. 28 (2H, m), 3. 18 (2H, s), 2. 97 (3H, s), 2. 72 (2H, m), 2. 56 (2H, m), 2. 36 (2H, m), 1. 52 (9H, s). Step 4:4 - (4 - (3 - (Methylsulfonyl)Propoxy)Phenyl) - 1, 2, 3, 6 -Tetrahydro pyridazinoneOf thymidine hydrochloride [thu[thu] - butyl 4 - (4 - (methylsulfonyl) phenyl) - 5, 6 - dihydropyridine -1 car diplopia [ley[ley] [thu[thu] (2H) - instead, the steps 3 [thu[thu] obtained in 4 - butyl - (4 - (3 - (methylsulfonyl) propoxy) phenyl) - 5, 6 - dihydro - (2H) but using car diplopia [ley[ley] [thu[thu] -1 pyridine, example 9 step 2 performs in a manner that said the same desired compound are obtained. 1 H NMR (400MHz, D2 O): δ 7. 34 (2H, d), 6. 85 (2H, d), 6. 00 (1H, s), 4. 12 (2H, s), 3. 28 (2H, m), 3. 18 (2H, s), 2. 97 (3H, s), 2. 72 (2H, m), 2. 56 (2H, m), 2. 36 (2H, m). <Manufacturing example 12>(3S) - ethyl 3 - (4 - (4 - (1 -Bromoethyl)Benzyloxy)Phenyl)[heyk[heyk]- 4 -The inosinateRotary cutter having the Step 1:1 - (4 - (Bromo methyl)Phenyl)1 one Manufacturing Nitrogen atmosphere, cap to [thol[thol] The reel ethane it comes 1 a-p - 5. 0g CCl a4 Dissolving NBS 14 is placed 100 ml agitating. 6g and AIBN 6. 7g in a enemy 0 °C-gate. 5 hours refluxing the reaction terminates chuck enemy MC and extraction of slowly heating the wire after washing the phosphorus, magnesium sulfate anhydride was dried and concentrated. Then, silica column chromatography separation desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 95 (2H, d), 7. 50 (2H, d), 4. 52 (2H, s), 2. 62 (3H, s). Step 2: (S)- ethyl 3 - (4 - (4 -Acetyl it cuts the qualitative jade city)Phenyl)[heyk[heyk]- 4 -Polycarbonate of inosinate Manufacturing 3 - ethyl - 2 obtained in the manufacturing example (S) (4 - hydroxyphenyl) - 4 - 1 - 1 obtained in the above steps polycarbonate [heyk[heyk] inosinate (4 - (bromo methyl) phenyl) ethane and superposed thereon but using, for example 6 step 2 performs in a manner that said the same desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 99 (2H, d), 7. 53 (2H, d) 7. 31 (2H, d), 6. 92 (2H, d), 5. 13 (2H, s), 4. 15 (2H, m), 4. 09 (1H, m), 2. 75 (2H, m), 2. 64 (3H, s), 1. 84 (3H, d), 1. 24 (3H, m). Step 3: (3S) - ethyl 3 - (4 - (4 - (1 - hydroxyethyl)Benzyloxy)Phenyl)[heyk[heyk]- 4 -InosinatePreparation of polycarbonate Nitrogen atmosphere, ethyl - 3 - (S) obtained in step 2 the cap (4 - (4 - acetyl it cuts the qualitative jade city) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate 1. 0g agitating a dissolving NaBH is placed THF 50 ml4 0. 16g in a enemy 0 °C-gate. 2 hours been rapidly at room temperature, the reaction terminates after washing and extraction of EA to chuck enemy slowly working phosphorus, magnesium sulfate anhydride and concentrated drying desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 8. 02 (2H, d), 7. 57 (2H, d) 7. 36 (2H, d), 6. 99 (2H, d), 5. 21 (2H, s), 4. 23 (2H, m), 4. 17 (1H, m), 3. 81 (1H, s), 2. 75 (2H, m), 2. 64 (3H, s), 1. 84 (3H, d), 1. 24 (3H, m). Step 4: (3S) - ethyl 3 - (4 - (4 - (1 -Bromoethyl)Benzyloxy)Phenyl)[heyk[heyk]- 4 -Inosinate methodProduced Nitrogen atmosphere, cap the step 3 obtained in (3S) - ethyl 3 - (4 - (4 - (1 - hydroxyethyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate 0. 76g dissolving triphenyl phosphate is placed MC 50 ml agitating a fine 0. 6g and CBr4 0. 75g in a enemy 0 °C-gate. 2 hours been rapidly at room temperature, the reaction terminates after washing and extraction of EA to chuck enemy slowly working phosphorus, magnesium sulfate anhydride and concentrated drying desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 8. 02 (2H, d), 7. 57 (2H, d) 7. 36 (2H, d), 6. 99 (2H, d), 5. 21 (2H, s), 4. 23 (2H, m), 4. 17 (1H, m), 3. 92 (1H, s), 2. 85 (2H, m), 2. 44 (3H, s), 1. 86 (3H, d), 1. 27 (3H, m). <Manufacturing example 13>2 - (piperazine - 1 - yl)Benzo[D] thiazole Of hydrochloride Manufacturing Step 1: [thu[thu]4 - butyl - (Benzo thiazole- 2 - yl) piperazine - 1 -car diplopia [ley[ley] [thu[thu] Manufacturing Nitrogen atmosphere, 2 - butyl piperazine - 1 - car diplopia [ley[ley] [thu[thu][thu[thu] to cap. 0g AN/DIPEA 2 agitating 100/50 ml distilled water dissolving the sugar syrup. In a 1 ml enemy 0 °C-gate. Then 2 - chloro-benzo thiazole 0. 9g after a the enemy it inflicted, 2 hours heating and refluxing, the reaction terminates after slowly the enemy it inflicted chuck, particularly as EA, after washing the working phosphorus, magnesium sulfate anhydride and concentrated drying desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 61 (1H, d), 7. 60 (1H, d), 7. 29 (1H, m), 7. 09 (1H, m), 3. 77 (4H, m), 2. 62 (4H, m), 1. 52 (9H, s). Step 2:2 - (piperazine - 1 - yl)Benzo[D] thiazole Of hydrochloride Manufacturing [thu[thu] - butyl 4 - (4 - (methylsulfonyl) phenyl) - 5, 6 - dihydropyridine -1 car diplopia [ley[ley] [thu[thu] - instead (2H), 4 - (benzo thiazol - 2 - yl) obtained in the above steps 1 - butyl [thu[thu]car diplopia [ley[ley] [thu[thu] but using piperazine - 1 -, example 9 step 2 performs in a manner that said the same desired compound are obtained. 1 H NMR (400MHz, D2 O): δ 7. 61 (1H, d), 7. 60 (1H, d), 7. 29 (1H, m), 7. 09 (1H, m), 3. 77 (4H, m), 2. 62 (4H, m). <Manufacturing example 14>2 - (piperazine - 1 - yl) - 5 -Propyl pyrimidineOf hydrochloride firstbath Step 1: [thu[thu]Butyl - 4 - (5 -Propyl pyrimidine- 2 - yl) piperazine - 1 -car diplopia [ley[ley] [thu[thu]Manufacturing 2 - chloro-benzo thiazole instead triazoles, 2 - chloro - 5 - propyl pyrimidine using but, example 13 step 1 performs in a manner equal to said desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 8. 19 (2H, s), 3. 77 (4H, m), 2. 62 (4H, m), 2. 41 (2H, m), 1. 61 (2H, m), 1. 52 (9H, s), 0. 96 (3H, m). Step 2:2 - (piperazine - 1 - yl) - 5 -Propyl pyrimidineOf hydrochloride Manufacturing [thu[thu] - butyl 4 - (4 - (methylsulfonyl) phenyl) - 5, 6 - dihydropyridine -1 car diplopia [ley[ley] [thu[thu] (2H) - instead, the steps 1 - butyl 4 - [thu[thu] obtained in (5 - propyl pyrimidine - 2 - yl) piperazine - 1 - car diplopia [ley[ley] [thu[thu] but using, example 9 step 2 performs in a manner that said the same desired compound are obtained. 1 H NMR (400MHz, D2 O): δ 8. 19 (2H, s), 3. 77 (4H, m), 2. 62 (4H, m), 2. 41 (2H, m), 1. 61 (2H, m), 0. 96 (3H, m). <Manufacturing example 15>6 - (piperazine - 1 - yl)knight reelroh mote nicotinateOf hydrochloride Manufacturing Step 1: [thu[thu]Butyl - 4 - (5 -Cyano roh blood [tin[tin]- 2 - yl) piperazine - 1 -car diplopia [ley[ley] [thu[thu] firstbath 2 - chloro-benzo thiazole instead triazoles, but using 6 - chloro the [ni[ni] the nose mote it plays but sprout work, example 13 step 1 performs in a manner equal to said desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 8. 41 (1H, s) 7. 61 (1H, d), 6. 59 (1H, d), 3. 77 (4H, m), 2. 62 (4H, m), 1. 52 (9H, s). Step 2:6 - (piperazine - 1 - yl)knight reelroh mote nicotinateOf hydrochloride Manufacturing [thu[thu] - butyl 4 - (4 - (methylsulfonyl) phenyl) - 5, 6 - dihydropyridine -1 car diplopia [ley[ley] [thu[thu] (2H) - instead, the steps 1 - butyl 4 - [thu[thu] obtained in (5 - cyano - 2 - yl roh blood [tin[tin]) piperazine - 1 - car diplopia [ley[ley] [thu[thu] but using, example 9 step 2 performs in a manner that said the same desired compound are obtained. 1 H NMR (400MHz, D2 O): δ 8. 41 (1H, s) 7. 61 (1H, d), 6. 59 (1H, d), 3. 77 (4H, m), 2. 62 (4H, m). <Manufacturing example 16>(S)- ethyl 3 - (4 - (4 - (2 - (Methyl opinion gun Neel jade city) Ethyl)Benzyloxy)Phenyl)[heyk[heyk]- 4 - hydroxybenzoate of inosinate Step 1:2 - (4 - (Bromo methyl)Phenyl) Ethanol Nitrogen atmosphere, cap to 2 - (4 - (bromo methyl) phenyl) THF 100 ml oh three tic[eyk[eyk] Hour [tu[tu] 5g and agitating a portion of the after dissolving, 0 °C - THF boranes in 70 ml enemy slowly a solution-gate. 2 time after stirring, the reaction terminates after the enemy it inflicted 0 °C temperatures slowly lowers the chuck, using extracted from the EA. The extracted organic layer reduced pressure drying desired compound are obtained. *1 H NMR (400MHz, CDCl3 ): Δ 37 (2H, d), 7. 24 (2H, d), 4. 51 (2H, s), 3. 89 (2H, m), 2. 89 (2H, m). Step 2: (S)- ethyl 3 - (4 - (4 - (2 - hydroxyethyl)Benzyloxy)Phenyl)[heyk[heyk]- 4 -The inosinateRotary cutter having the (4 - (bromo methyl) phenyl) methanol instead, obtained in the step 1 2 - (4 - (bromo methyl) phenyl) ethanol but, example 6 step 2 performs in a manner that said the same desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 40 (2H, d), 7. 30 (2H, d), 7. 27 (2H, d), 6. 95 (2H, d), 5. 04 (2H, s), 4. 18 (2H, m), 4. 11 (1H, m), 3. 89 (2H, m), 2. 91 (2H, m), 2. 71 (2H, m), 1. 84 (3H, s), 1. 38 (1H, m), 1. 25 (3H, m). *Step 3: (S)- ethyl 3 - (4 - (4 - (2 - (Methyl opinion gun Neel jade city) Ethyl)Benzyloxy)Phenyl)[heyk[heyk]- 4 -Polycarbonate of inosinate Ethyl 3 - (4 - (4 - (hydroxymethyl) benzyloxy) phenyl) - 4 - formation [heyk[heyk] inosinate instead, obtained in the above steps 2 - (S) ethyl 3 - (4 - (4 - (2 - hydroxyethyl) benzyloxy) phenyl) - 4 - inosinate [heyk[heyk] formation but using, performs in a manner that said desired compound example 3 step equal to 6 are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 40 (2H, d), 7. 30 (2H, d), 7. 27 (2H, d), 6. 95 (2H, d), 5. 04 (2H, s), 4. 18 (2H, m), 4. 11 (1H, m), 3. 99 (2H, m), 2. 95 (3H, s), 2. 93 (2H, m), 2. 71 (2H, m), 1. 84 (3H, s), 1. 25 (3H, m). <Embodiments 1>3 - (4 - (3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)Benzyloxy)[...]Carbonyl) - 4 - of the tooth [heyk[heyk] inosinate Step 1: ethyl 3 - (4 - (3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)Benzyloxy)Phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] of inosinate Nitrogen atmosphere, 500 ml cap for the preparation of example 4 has been produced (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) phenyl) methanol (19. 54 g) and tetrahydrofuran mixable (80 ml) which can be after dissolving agitating, made in manufacturing example 1 ethyl 3 - (4 - hydroxyphenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate (18. 42 g) and tri-phenyl phosphine (31. 21 g) was added slowly a. Then, isopropyl azo die car luck thread [ley[ley] [thu[thu] die 0 °C (23. 4 ml) slowly dripped into stirring is increased by 4 hours using a dropping funnel at ordinary temperature-gate. The reaction terminates distilled water (200 ml) enemy evaporants and ethyl acetate (300 ml) are extracted using, extracted organic layer reduced pressure drying target compound (32. 1 g, 87. 9%) by Congress. 1 H NMR (400MHz, CDCl3 ): Δ 7. 46 (1H, s), 7. 31 (5H, m), 6. 93 (2H, d), 6. 02 (1H, m), 5. 04 (2H, s), 4. 13 (2H, m), 4. 08 (1H, m), 4. 04 (4H, s), 2. 69 (4H, m), 2. 49 (2H, s), 1. 94 (2H, t), 1. 84 (3H, d), 1. 31 (3H, t). Step 2:3 - (4 - (3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)Benzyloxy)Phenyl)Inosinate [heyk[heyk] - 4 - of the tooth Nitrogen atmosphere, ethyl 3 - 1 the cap 500 ml made in step (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate (32. 1 g), methanol (50 ml) and distilled water (50 ml) which can reduce agitating after dissolving, potassium hydroxide at room temperature (19. 5 g) adding evaporants stirring-gate 1 hour or more. The reaction terminates, a 1M aqueous hydrochloric acid solution to pH 2 to 3 and the using an acidic ethyl acetate (300 ml) to extract the target compound using dry (24. 1 g, 79. 9%) by Congress. 1 H NMR (400MHz, CDCl3 ): Δ 7. 44 (1H, s), 7. 34 (5H, m), 6. 91 (2H, d), 6. 00 (1H, t), 5. 02 (2H, s), 4. 08 (1H, m), 4. 04 (4H, s), 2. 73 (4H, m), 2. 48 (2H, s), 1. 92 (2H, t), 1. 82 (3H, s). <Embodiments 2>3 - (4 - (3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)Benzyloxy)[...]Carbonyl) - 4 - tooth [heyk[heyk] inosinate L - lysine salt Nitrogen atmosphere, made in the embodiments 3 - 1 500 ml flasks (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - tooth [heyk[heyk] inosinate (24. 1 g) and ethanol (170 ml) which can be after dissolving agitating, L - lysine (7. 33 g) some excellent. Then, to the reaction temperature 50 °C temperature 50 °C by stirring in 30 minutes, 30 minutes stirring the cooling back-gate. The end point of the reaction, resulting solids contained in desired compound (31. 5 g, 73. 3%) by Congress. 1 H NMR (400MHz, D2 O): δ 7. 11 (3H, m), 6. 99 (3H, m), 6. 64 (2H, d), 5. 65 (1H, s), 4. 59 (2H, s), 3. 79 (5H, s), 3. 60 (1H, t), 2. 88 (2H, t), 2. 35 (2H, d), 2. 23 (2H, s), 2. 14 (2H, s), 1. 75 (2H, m), 1. 59 (7H, m), 1. 38 (2H, m). <Embodiments 3>4 - (4 - (3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)Benzyloxy)[...]Carbonyl) - 4 - of the tooth [heyk[heyk] inosinate Step 1: ethyl 4 - (4 - (3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)Benzyloxy)Phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] of inosinate Nitrogen atmosphere, made in manufacturing the cap 100 ml 5 example (4 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) phenyl) methanol (1. 5 g) and tetrahydrofuran mixable (20 ml) which can be after dissolving agitating, made in example 1 said ethyl 3 - (4 - hydroxyphenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate (1. 41 g) and tri-phenyl phosphine (2. 39 g) was added slowly a. Then, isopropyl azo die car luck thread [ley[ley] [thu[thu] die 0 °C (9. 38 ml) slowly dripped into stirring is increased by 4 hours using a dropping funnel at ordinary temperature-gate. The reaction terminates distilled water (50 ml) and enemy evaporants ethyl acetate (100 ml) are extracted using, extracted organic layer reduced pressure drying target compound (1. 38 g, 49. 2%) by Congress. 1 H NMR (400MHz, CDCl3 ): Δ 7. 42 (2H, d), 7. 37 (2H, d), 7. 30 (2H, d), 6. 92 (2H, d), 6. 01 (1H, s), 5. 01 (2H, s), 4. 14 (2H, m), 4. 06 (5H, m), 2. 70 (4H, m), 2. 49 (2H, s), 1. 94 (2H, t), 1. 84 (3H, d), 1. 24 (3H, t). Step 2:4 - (4 - (3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)Benzyloxy)Phenyl)Inosinate [heyk[heyk] - 4 - of the tooth Nitrogen atmosphere, step 1 ethyl 4 - made in the cap 500 ml (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate (1. 38 g), methanol (10 ml) and distilled water (10 ml) which can be after dissolving agitating, potassium hydroxide at room temperature (1. 25 g) adding evaporants stirring-gate 1 hour or more. The reaction terminates, a 1M aqueous hydrochloric acid solution to pH 2 to 3 and the using an acidic ethyl acetate (50 ml) to extract the target compound using dry (0. 98 g, 75. 6%) by Congress. 1 H NMR (400MHz, CDCl3 ): Δ 7. 41 (2H, d), 7. 36 (2H, d), 7. 29 (2H, d), 6. 92 (2H, d), 6. 01 (1H, s), 5. 01 (2H, s), 4. 04 (5H, m), 2. 77 (4H, m), 2. 49 (2H, s), 1. 96 (2H, t), 1. 83 (3H, d). <Embodiments 4>3 - (4 - (3 - (4 -Oxo gap claw [heyk[heyk]- 1 -Enyl)Benzyloxy)Phenyl)[heyk[heyk]- 4 -InosinateOf the tooth Nitrogen atmosphere, made in the embodiments 1 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - (1 g) the tooth [heyk[heyk] inosinate and tetrahydrofuran mixable (5 ml) which can be after dissolving agitating, 6N hydrochloric acid aqueous solution (5 ml) at room temperature stirring-gate by adding 1 hour or more. The reaction terminates distilled water (50 ml) and enemy evaporants ethyl acetate (50 ml) are extracted using, extracted organic layer reduced pressure drying target compound (0. 76 g, 84. 6%) by Congress. 1 H NMR (400MHz, CDCl3 ): Δ 7. 48 (1H, s), 7. 40 (5H, m), 6. 94 (2H, d), 6. 13 (1H, s), 5. 07 (2H, s), 4. 05 (1H, m), 3. 10 (1. 5H, t), 2. 93 (1. 5H, t), 2. 82 (2H, m), 2. 67 (2H, t), 1. 85 (3H, s). <Embodiments 5>3 - (4 - (3 - (4 -Hydroxy current events claw [heyk[heyk]- 1 -Enyl)Benzyloxy)Phenyl)[heyk[heyk]-4 - tooth of inosinate Nitrogen atmosphere, 100 ml cap the embodiments made in 3 - 4 (4 - (3 - (4 - oxo - 1 - enyl gap claw [heyk[heyk]) benzyloxy) phenyl) - 4 - (1 g) the tooth [heyk[heyk] inosinate and ethanol (10 ml) which can be after dissolving agitating, sodium preferred embodiment (0. 3 g) is added 3 hours stirring at room temperature-gate. The reaction terminates, an aqueous hydrochloric acid solution to pH 4 to 5 using a 1N acidification and ethyl acetate (100 ml) and distilled water (100 ml) extracted from the using. The extracted organic layer reduced pressure drying target compound (0. 81 g, 80. 6%) by Congress. 1 H NMR (400MHz, CDCl3 ): Δ 7. 44 (1H, s), 7. 33 (5H, m), 6. 93 (2H, d), 6. 02 (1H, s), 5. 03 (2H, s), 4. 08 (2H, s), 2. 78 (2H, m), 2. 55 (2. 5H, m), 2. 22 (1H, m), 2. 04 (1H, m), 1. 85 (3H, s). <Embodiments 6>3 - (4 - (3 - (4 -Hydroxy current events claw [heyk[heyk]- 1 -Enyl)Benzyloxy)Phenyl)[heyk[heyk]-4 - tooth inosinate L - lysine salt Nitrogen atmosphere, 100 ml cap the embodiments made in 3 - 5 (4 - (3 - (4 - hydroxy - 1 - enyl current events claw [heyk[heyk]) benzyloxy) phenyl) - 4 - (1 g) the tooth [heyk[heyk] inosinate and ethanol (170 ml) which can be after dissolving agitating, L - lysine (0. 7 g) some excellent. Then, to the reaction temperature 50 °C temperature 50 °C by stirring in 30 minutes, 30 minutes stirring the cooling back-gate. The end point of the reaction, resulting solids contained in desired compound (0. 95 g, 69. 1%) by Congress. 1 H NMR (400MHz, D2 O): δ 7. 11 (3H, m), 6. 99 (3H, m), 6. 64 (2H, d), 5. 65 (1H, s), 4. 59 (2H, s), 3. 79 (1H, s), 3. 60 (1H, t), 2. 88 (2H, t), 2. 35 (2H, d), 2. 23 (2H, s), 2. 14 (2H, s), 1. 75 (2H, m), 1. 59 (7H, m), 1. 38 (2H, m). <Embodiments 7>(3S) - 3 - (4 - (3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)Benzyl jadeIn) phenyl) - 4 - of the tooth [heyk[heyk] inosinate Step 1: ethyl - (3S) - 3 - (4 - (3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)BenzylOxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] of inosinate Nitrogen atmosphere, made in manufacturing the cap 500 ml 4 example (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) phenyl) methanol (19. 54 g) and tetrahydrofuran mixable (80 ml) which can be after dissolving agitating, ethyl - 3 - (S) made in the manufacturing example 2 (4 - hydroxyphenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate (18. 42 g) and tri-phenyl phosphine (31. 21 g) was added slowly a. Then, isopropyl azo die car luck thread [ley[ley] [thu[thu] die 0 °C (23. 4 ml) slowly dripped into stirring is increased by 4 hours using a dropping funnel at ordinary temperature-gate. The reaction terminates distilled water (200 ml) enemy evaporants and ethyl acetate (300 ml) are extracted using, extracted organic layer reduced pressure drying desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 46 (1H, s), 7. 31 (5H, m), 6. 93 (2H, d), 6. 02 (1H, m), 5. 04 (2H, s), 4. 13 (2H, m), 4. 08 (1H, m), 4. 04 (4H, s), 2. 69 (4H, m), 2. 49 (2H, s), 1. 94 (2H, t), 1. 84 (3H, d), 1. 31 (3H, t). Step 2: (3S) - 3 - (4 - (3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)Benzyloxy)Phenyl) - 4 - of the tooth [heyk[heyk] inosinate Nitrogen atmosphere, made in the step 1 - 500 ml flasks ethyl (3S) - 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate (32. 1 g), methanol (50 ml) and distilled water (50 ml) which can reduce agitating after dissolving, potassium hydroxide at room temperature (19. 5 g) adding evaporants stirring-gate 1 hour or more. The reaction terminates, a 1M aqueous hydrochloric acid solution to pH 2 to 3 and the using an acidic ethyl acetate (300 ml) to extract the target compound using dry (24. 1 g, 66. 2%) by Congress. 1 H NMR (400MHz, CDCl3 ): Δ 7. 44 (1H, s), 7. 34 (5H, m), 6. 91 (2H, d), 6. 00 (1H, t), 5. 02 (2H, s), 4. 08 (1H, m), 4. 04 (4H, s), 2. 73 (4H, m), 2. 48 (2H, s), 1. 92 (2H, t), 1. 82 (3H, s). <Embodiments 8>(3R) - 3 - (4 - (3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)Benzyl jadeIn) phenyl) - 4 - of the tooth [heyk[heyk] inosinate Step 1: ethyl - (3R) - 3 - (4 - (3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)BenzylOxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] of inosinate Nitrogen atmosphere, made in manufacturing the cap 500 ml 4 example (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) phenyl) methanol (19. 54 g) and tetrahydrofuran mixable (80 ml) which can be after dissolving agitating, (R)- ethyl 3 - made in the manufacturing example 3 (4 - hydroxyphenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate (18. 42 g) and tri-phenyl phosphine (31. 21 g) was added slowly a. Then, isopropyl azo die car luck thread [ley[ley] [thu[thu] die 0 °C (23. 4 ml) slowly dripped into stirring is increased by 4 hours using a dropping funnel at ordinary temperature-gate. The reaction terminates distilled water (200 ml) enemy evaporants and ethyl acetate (300 ml) are extracted using, extracted organic layer reduced pressure drying desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 46 (1H, s), 7. 31 (5H, m), 6. 93 (2H, d), 6. 02 (1H, m), 5. 04 (2H, s), 4. 13 (2H, m), 4. 08 (1H, m), 4. 04 (4H, s), 2. 69 (4H, m), 2. 49 (2H, s), 1. 94 (2H, t), 1. 84 (3H, d), 1. 31 (3H, t). Step 2: (3R) - 3 - (4 - (3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)Benzyloxy)Phenyl) - 4 - of the tooth [heyk[heyk] inosinate Nitrogen atmosphere, made in the step 1 - 500 ml flasks ethyl (3R) - 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate (32. 1 g), methanol (50 ml) and distilled water (50 ml) which can reduce agitating after dissolving, potassium hydroxide at room temperature (19. 5 g) adding evaporants stirring-gate 1 hour or more. The reaction terminates, a 1M aqueous hydrochloric acid solution to pH 2 to 3 and the using an acidic ethyl acetate (300 ml) to extract the target compound using dry (17. 3 g, 47. 5%) by Congress. 1 H NMR (400MHz, CDCl3 ): Δ 7. 44 (1H, s), 7. 34 (5H, m), 6. 91 (2H, d), 6. 00 (1H, t), 5. 02 (2H, s), 4. 08 (1H, m), 4. 04 (4H, s), 2. 73 (4H, m), 2. 48 (2H, s), 1. 92 (2H, t), 1. 82 (3H, s). <Embodiments 9>(3S) - 3 - (4 - (3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)Benzyl jadeIn) phenyl) - 4 - tooth [heyk[heyk] inosinate L - lysine salt Nitrogen atmosphere, made in 500 ml cap the embodiments 7 (3S) - 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - tooth [heyk[heyk] inosinate (24. 1 g) and ethanol (170 ml) which can be after dissolving agitating, L - lysine (7. 33 g) some excellent. Then, to the reaction temperature 50 °C temperature 50 °C by stirring in 30 minutes, 30 minutes stirring the cooling back-gate. The end point of the reaction, resulting solids contained in desired compound (22. 5 g, 69. 8%) by Congress. 1 H NMR (400MHz, D2 O): δ 7. 11 (3H, m), 6. 99 (3H, m), 6. 64 (2H, d), 5. 65 (1H, s), 4. 59 (2H, s), 3. 79 (5H, s), 3. 60 (1H, t), 2. 88 (2H, t), 2. 35 (2H, d), 2. 23 (2H, s), 2. 14 (2H, s), 1. 75 (2H, m), 1. 59 (7H, m), 1. 38 (2H, m). <Embodiments 10>(3R) - 3 - (4 - (3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)BenzylOxy) phenyl) - 4 - tooth [heyk[heyk] inosinate L - lysine salt Nitrogen atmosphere, made in 500 ml cap the embodiments 8 (3R) - 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - tooth [heyk[heyk] inosinate (24. 1 g) and ethanol (170 ml) which can be after dissolving agitating, L - lysine (7. 33 g) some excellent. Then, to the reaction temperature 50 °C temperature 50 °C by stirring in 30 minutes, 30 minutes stirring the cooling back-gate. The end point of the reaction, resulting solids contained in desired compound (16. 2 g, 71. 4%) by Congress. 1 H NMR (400MHz, D2 O): δ 7. 11 (3H, m), 6. 99 (3H, m), 6. 64 (2H, d), 5. 65 (1H, s), 4. 59 (2H, s), 3. 79 (5H, s), 3. 60 (1H, t), 2. 88 (2H, t), 2. 35 (2H, d), 2. 23 (2H, s), 2. 14 (2H, s), 1. 75 (2H, m), 1. 59 (7H, m), 1. 38 (2H, m). <Embodiments 11>(3S) - 3 - (4 - (3 - (1, 4 -[4, 5] oxa cyber die to a process for the- 7 - en - 8 - yl)BenzylOxy) phenyl) - 4 - tooth [heyk[heyk] inosinate sodium salt Nitrogen atmosphere, made in 500 ml cap the embodiments 7 (3S) - 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - (1 g) the tooth [heyk[heyk] inosinate and ethanol (170 ml) which can be after dissolving agitating, 3N sodium hydroxide aqueous solution (0. 77 ml) enemy a-gate. Then, the end point of the reaction stirring at room temperature, then cream a reaction solution, isopropyl alcohol (10 ml) is added such that the resulting solids contained in desired compound (0. 73 g, 69. 2%) by Congress. 1 H NMR (400, CDCl3 ): Δ 7. 44 (1H, s), 7. 34 (5H, m), 6. 91 (2H, d), 6. 00 (1H, t), 5. 02 (2H, s), 4. 08 (1H, m), 4. 04 (4H, s), 2. 73 (4H, m), 2. 48 (2H, s), 1. 92 (2H, t), 1. 82 (3H, s) <Embodiments 12>3 - (4 - (4 - ((3, 4 -Dihydroisoquinoline-2 (1H) - one)Methyl)Benzyloxy) Phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing Step 1: ethyl 3 - (4 - (4 - ((3, 4 -Dihydroisoquinoline-2 (1H) - one)Methyl)Benzyl jadeIn) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] of inosinate Nitrogen atmosphere, cap to 1, 2, 3, 4 - tetrahydro isoquinoline 0. 5g agitating and injecting a DMF 20 ml after dissolving, purification of cesium 1 at room temperature. 2g been applying. 30 minutes, 6 obtained in the manufacturing example ethyl 3 - (4 - (4 - ((methyl opinion gun Neel jade city) methyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate 1. 0 g behind a the enemy it inflicted, stirring time-gate 12 at room temperature. The reaction terminates (brine) then ethyl acetate slowly the enemy it inflicted chuck by extracting magnesium sulfate anhydride and concentrated drying after washing the knife of his. Then, silica column chromatography separation desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 38 (2H, d), 7. 31 (2H, d), 7. 22 (2H, d), 7. 16 (3H, m), 6. 97 (3H, m), 4. 98 (2H, s), 4. 14 (2H, m), 4. 09 (1H, s), 3. 91 (1H, d), 3. 70 (3H, m), 2. 92 (4H, s), 2. 73 (2H, m), 1. 83 (3H, s), 1. 29 (3H, m). Step 2:3 - (4 - (4 - ((3, 4 -Dihydroisoquinoline-2 (1H) - one)Methyl)Benzyloxy) [...]Carbonyl) - 4 - [heyk[heyk] inosinate [eyk[eyk] Hour [tu[tu] of wing Nitrogen atmosphere, ethyl 3 - made in step 1 the cap (4 - (4 - ((3, 4 - dihydroisoquinoline -2 (1H) - yl) methyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate 0. 7g THF a, which can be after dissolving methanol and chuck agitating, 0 lithium hydroxide at room temperature. 7g-gate slowly adding a stirring 1 hour or more. The reaction terminates, a 1M aqueous hydrochloric acid solution to pH 2 to 3 and the extracted using ethyl acetate using an acidic and reduced pressure drying target compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 38 (2H, d), 7. 31 (2H, d), 7. 22 (2H, d), 7. 16 (3H, m), 6. 97 (3H, m), 4. 98 (2H, s), 4. 09 (1H, s), 3. 91 (1H, d), 3. 70 (3H, m), 2. 92 (4H, s), 2. 73 (2H, m), 1. 83 (3H, s). <Embodiments 13>3 - (4 - (3 -1 fragrance- 4 - ((3, 4 -Dihydroisoquinoline-2 (1H) -Yl) methyl) benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing Step 1: ethyl 3 - (4 - (3 -1 fragrance- 4 - ((3, 4 -Dihydroisoquinoline-2 (1H) -Yl) methyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] of inosinate Nitrogen atmosphere, cap to (3 - 1 - 4 - ((3, 4 - dihydroisoquinoline -2 (1H) - yl) methyl) phenyl fragrance) methanol 1. 0g which can be after dissolving 30 ml tetrahydrofuran mixable and agitating, obtained in the manufacturing example 1 ethyl 3 - (4 - hydroxyphenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate 0. 8g and tri phenyl phosphine 0. 6g a was slowly added. Then, isopropyl azo die car luck thread [ley[ley] [thu[thu] 0 0 °C die. 5 ml by 4 hours using a dropping funnel slowly dripped into stirring-gate is increased at ordinary temperature. The reaction terminates after enemy evaporants extracted using distilled water and ethyl acetate, extracted organic layer reduced pressure drying desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 12. 56 (1H, s), 8. 26 (1H, d), 7. 43 (2H, d), 7. 25 (6H, m), 7. 21 (1H, d), 7. 02 (1H, d), 6. 89 (2H, d), 5. 46 (1H, s), 5. 03 (2H, s), 4. 14 (2H, m), 4. 05 (1H, s), 3. 92 (1H, s), 3. 70 (1H, s), 3. 35 (1H, s), 3. 27 (1H, s), 3. 03 (1H, s), 2. 83 (2H, m), 2. 01 (4H, m), 1. 84 (3H, d), 1. 51 (4H, m), 1. 29 (3H, m). Step 2:3 - (4 - (3 -1 fragrance- 4 - ((3, 4 -Dihydroisoquinoline-2 (1H) - one)Methyl) benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing Ethyl 3 - (4 - (4 - ((3, 4 - dihydroisoquinoline -2 (1H) - yl) methyl) benzyloxy) phenyl) - 4 - formation [heyk[heyk] inosinate instead, ethyl 3 - (4 - (3 - 1 - 4 - ((3, 4 - dihydroisoquinoline -2 (1H) - yl) methyl) fragrance-benzyloxy) phenyl) - 4 - inosinate [heyk[heyk] formation but using, the embodiments 12 steps 2 performs in a manner such as desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 12. 56 (1H, s), 8. 26 (1H, d), 7. 43 (2H, d), 7. 25 (6H, m), 7. 21 (1H, d), 7. 02 (1H, d), 6. 89 (2H, d), 5. 46 (1H, s), 5. 03 (2H, s), 4. 05 (1H, s), 3. 92 (1H, s), 3. 70 (1H, s), 3. 35 (1H, s), 3. 27 (1H, s), 3. 03 (1H, s), 2. 83 (2H, m), 2. 01 (4H, m), 1. 84 (3H, d), 1. 51 (4H, m). *<Embodiments 14>3 - (4 - (4 - ((4 -Phenyl- 5, 6 -Dihydropyridine-1 (2H) - yl)Methyl) BenzylOxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetra high draw cow quinoline instead, 4 - phenyl - 1, 2, 3, 6 - tetrahydropyridine hydrochloride using but, the embodiments 12 performs in a manner such as desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 25 (2H, d), 6. 78 (2H, d), 4. 95 (1H, s), 4. 14 (2H, m), 4. 04 (1H, m), 2. 68 (2H, m), 1. 84 (3H, d), 1. 29 (3H, t). <Embodiments 15>3 - (4 - (4 - ((4 -Phenyl piperazine- 1 - yl)Methyl)Benzyloxy)Phenyl)[heyk[heyk]- 4 -theroh [ik[ik][eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, but using 1 - phenyl blood lung position, the embodiments 12 performs in a manner identical to the desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 37 (2H, d), 7. 29 (4H, m), 7. 11 (2H, d), 6. 93 (5H, m), 4. 96 (2H, s), 4. 13 (1H, s), 3. 66 (2H, m), 3. 23 (4H, s), 2. 83 (2H, m), 2. 66 (2H, s), 1. 82 (3H, s). <Embodiments 16>3 - (4 - (4 - ((6 -Methoxy- 3, 4 -Dihydroisoquinoline-2 (1H) - one)Methyl) Benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, 6 - methoxy - 1, 2, 3, 4 - tetrahydro isoquinoline using said obtained in example 8 but, the embodiments 12 performs in a manner identical to the desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 40 (4H, q), 7. 26 (2H, d), 6. 92 (3H, q), 6. 66 (2H, d), 5. 06 (2H, s), 3. 94 (1H, s), 3. 73 (3H, s), 3. 63 (2H, s), 3. 35 (3H, s), 2. 78 (2H, t), 2. 62 (2H, t), 2. 58 (2H, s), 1. 77 (3H, s) <Embodiments 17>3 - (4 - (4 - ((4 -Phenylpiperidine- 1 - yl)Methyl)Benzyloxy)Phenyl)[heyk[heyk]- 4 -theroh [ik[ik][eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, but using 4 - phenylpiperidine, the embodiments 12 performs in a manner identical to the desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 44 (2H, d), 7. 32 (2H, d), 7. 23 (5H, t), 7. 13 (2H, d), 6. 96 (2H, d), 4. 92 (2H, s), 4. 16 (1H, s), 3. 85 (2H, q), 3. 33 (2H, t), 2. 90 (1H, d), 2. 78 (1H, m), 2. 58 (1H, t), 2. 38 (2H, t), 2. 02 (2H, m), 1. 83 (5H, m). <Embodiments 18>3 - (4 - (4 - ((4 - (4 -Fluorophenyl) Piperazine - 1 - yl)Methyl)Benzyloxy) [...]Carbonyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, 1 - (4 - fluorophenyl) piperazine using modified but, the embodiments 12 performs in a manner identical to the desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 60 (2H, d), 7. 46 (2H, d), 7. 30 (3H, d), 6. 97 (2H, t), 6. 86 (4H, m), 5. 01 (2H, s), 4. 21 (2H, s), 4. 04 (1H, t), 3. 50 (4H, d), 3. 25 (4H, s), 2. 78 (2H, m), 1. 80 (3H, d). <Embodiments 19>3 - (4 - (4 - ((4 - (4 - (Trifluoromethyl)Phenyl) Piperazine - 1 - yl)Methyl) Benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, 1 - (4 - (trifluoromethyl) phenyl) piperazine using modified but, the embodiments 12 performs in a manner identical to the desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 63 (2H, d), 7. 51 (4H, d), 7. 21 (2H, d), 6. 93 (2H, d), 6. 74 (2H, s), 5. 03 (2H, s), 4. 13 (2H, m), 4. 01 (1H, t), 3. 73 (4H, s), 2. 96 (4H, s), 2. 71 (2H, m), 1. 78 (3H, s). <Embodiments 20>3 - (4 - (4 - ((4 - (4 - (3 - (Methylsulfonyl)Propoxy)Phenyl) Piperazine - 1 - yl)Methyl) benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, 1 - (4 - (3 - (methylsulfonyl) propoxy) phenyl) piperazine hydrochloride using but, the embodiments the test compound 12 identical to the method are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 65 (2H, d), 7. 49 (2H, d), 7. 30 (2H, d), 6. 87 (6H, m), 5. 07 (2H, s), 4. 20 (2H, d), 4. 08 (2H, t), 4. 01 (1H, t), 6. 63 (2H, s), 3. 49 (4H, m), 3. 26 (2H, t), 3. 01 (2H, s), 2. 97 (3H, s), 2. 71 (2H, m), 2. 34 (2H, m), 1. 83 (2H, d). <Embodiments 21>(S)- 3 - (4 - (4 - ((3, 4 -Dihydroisoquinoline-2 (1H) - one)Methyl) BenzylOxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing Step 1: ethyl (S)- 3 - (4 - (4 - ((3, 4 -Dihydroisoquinoline-2 (1H) - one)Methyl)BenzylOxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] of inosinate * Nitrogen atmosphere, cap to 1, 2, 3, 4 - tetrahydro isoquinoline 0. 5g agitating and injecting a DMF 20 ml after dissolving, purification of cesium 1 at room temperature. 1g been applying. 30 minutes (S) 3 - ethyl - 7 obtained in the manufacturing example (4 - (4 - ((methyl opinion gun Neel jade city) methyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate 1. 0g the enemy it inflicted behind a, 12 at room temperature stirring time-gate. The reaction terminates in distilled water (brine) then ethyl acetate the enemy it inflicted evaporants knife of magnesium sulfate anhydride is carried out by extracting wash after drying was and concentrated. Then, silica column chromatography separation desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 38 (2H, d), 7. 31 (2H, d), 7. 22 (2H, d), 7. 16 (3H, m), 6. 97 (3H, m), 4. 98 (2H, s), 4. 14 (2H, m), 4. 09 (1H, s), 3. 91 (1H, d), 3. 70 (3H, m), 2. 92 (4H, s), 2. 73 (2H, m), 1. 83 (3H, s), 1. 29 (3H, m). Step 2: (S)- 3 - (4 - (4 - ((3, 4 -Dihydroisoquinoline-2 (1H) - one)Methyl) Benzyl jadeIn) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing * Nitrogen atmosphere, step 1 ethyl - 3 - (S) made in the cap (4 - (4 - ((3, 4 - dihydroisoquinoline -2 (1H) - yl) methyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate 0. 5g a THF, methanol and distilled water is charged into a agitating after dissolving, 0 lithium hydroxide at room temperature. 5g 1 hours stirring slowly adding a-gate. The reaction terminates, a 1M aqueous hydrochloric acid solution to pH 2 to 3 and the extracted using ethyl acetate using an acidic and reduced pressure drying target compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 38 (2H, d), 7. 31 (2H, d), 7. 22 (2H, d), 7. 16 (3H, m), 6. 97 (3H, m), 4. 98 (2H, s), 4. 09 (1H, s), 3. 91 (1H, d), 3. 70 (3H, m), 2. 92 (4H, s), 2. 73 (2H, m), 1. 83 (3H, s). <Embodiments 22>(S)- 3 - (4 - (4 - ((4 - (4 - (Trifluoromethyl)Phenyl) Piperazine - 1 - yl)melterCefuroxime) benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, 1 - (4 - (trifluoromethyl) phenyl) piperazine using modified but, the embodiments 21 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 65 (2H, d), 7. 51 (4H, m), 7. 30 (2H, d), 6. 61 (2H, d), 6. 85 (2H, d), 5. 05 (2H, s), 4. 21 (2H, s), 4. 03 (1H, t), 3. 68 (4H, s), 3. 49 (2H, s), 2. 84 (2H, s), 2. 70 (2H, m), 1. 82 (3H, s). <Embodiments 23>(S)- 3 - (4 - (4 - ((4 - (4 -Fluorophenyl) Piperazine - 1 - yl)Methyl)Benzyl jadeIn) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, 1 - (4 - fluorophenyl) piperazine using modified but, the embodiments the test compound in the same 21 are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 39 (2H, d), 7. 30 (2H, d), 7. 19 (2H, d), 6. 96 (4H, m), 6. 87 (2H, m), 4. 97 (2H, s), 4. 10 (2H, s), 3. 81 (1H, d), 3. 51 (1H, d), 3. 15 (4H, s), 2. 80 (6H, m), 1. 82 (3H, s). <Embodiments 24>potassium (S)- 3 - (4 - (4 - ((4 - (4 - (Trifluoromethyl)Phenyl) - Piperazine1 - yl) methyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] of inosinate Nitrogen atmosphere, cap the embodiments made in 23 (S)- 3 - (4 - (4 - ((4 - (4 - fluorophenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate 0. 4g and ethanol which can be after dissolving 10 ml agitating, 3N potassium hydroxide aqueous solution 0. A 3 ml enemy-gate. Then, the end point of the reaction stirring at room temperature, then cream a reaction solution, filtering by adding isopropyl alcohol resulting solids desired compound are obtained. *1 H NMR (400MHz, D2 O): δ 7. 10 (4H, m), 6. 98 (2H, d), 6. 57 (4H, d), 6. 38 (2H, s), 4. 55 (2H, s), 3. 82 (1H, s), 3. 07 (2H, s), 2. 59 (4H, s), 2. 36 (2H, s), 2. 13 (4H, s), 1. 51 (3H, s). <Embodiments 25>(S)- 3 - (4 - (4 - ((6 -Methoxy- 3, 4 -Dihydroisoquinoline-2 (1H) - one)melterCefuroxime) benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, 6 - methoxy - 1, 2, 3, 4 - tetrahydro isoquinoline using but, the embodiments 21 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, DMSO): δ 7. 40 (4H, q), 7. 26 (2H, d), 6. 94 (3H, m), 6. 68 (2H, m), 5. 06 (2H, s), 3. 95 (1H, t), 3. 70 (3H, s), 3. 51 (2H, s), 3. 43 (2H, s), 2. 77 (2H, t), 2. 66 (2H, t), 2. 57 (2H, d), 1. 75 (3H, d). <Embodiments 26>(S)- 3 - (4 - (4 - ((4 -Phenylpiperidine- 1 - yl)Methyl)Benzyloxy)Phenyl)[heyk[heyk]-4 - inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, but using 4 - phenylpiperidine, the embodiments 21 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 66 (2H, d), 7. 49 (2H, d), 7. 30 (7H, m), 6. 87 (2H, d), 5. 04 (2H, s), 4. 19 (2H, s), 4. 06 (1H, t), 3. 59 (2H, d), 2. 73 (7H, m), 2. 00 (2H, d), 1. 82 (3H, s). <Embodiments 27>(S)- 3 - (4 - (4 - (Isoindoline- 2 -Ylmethyl)Benzyloxy)Phenyl)[heyk[heyk]- 4 -InosinateWing [eyk[eyk] Hour [tu[tu] manufacturing The 1, 2, 3, 4 - tetrahydro isoquinoline instead, using isocyanate indole phosphorus but, the embodiments 21 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 68 (2H, d), 7. 47 (2H, d), 7. 38 (2H, m), 7. 30 (4H, m), 6. 87 (2H, d), 5. 06 (2H, s), 4. 90 (2H, s), 4. 32 (4H, m), 4. 05 (1H, t), 2. 81 (2H, m), 1. 83 (3H, s). <Embodiments 28>(S)- 3 - (4 - (4 - ((4 -Phenyl- 5, 6 -Dihydropyridine-1 (2H) - yl)Methyl)togetherqualitative jade city) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, 4 - phenyl - 1, 2, 3, 6 - tetrahydropyridine hydrochloride using but, the embodiments 21 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 47 (2H, d), 7. 36 (9H, m), 6. 88 (2H, d), 5. 99 (1H, s), 4. 99 (2H, s), 4. 18 (1H, m), 4. 06 (2H, m), 3. 53 (2H, s), 3. 22 (2H, s), 2. 82 (4H, m), 1. 82 (3H, s). <Embodiments 29>(S)- 3 - (4 - (4 - ((4 - (4 - (maul [thok[thok] hour maul [thok[thok] city)Phenyl) Piperazine - 1 - yl)Methyl)togetherqualitative jade city) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, 1 - (4 - (maul [thok[thok] hour maul [thok[thok] city) phenyl) piperazine using modified but, the embodiments 21 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 57 (2H, d), 7. 46 (2H, d), 7. 26 (2H, d), 6. 97 (2H, d), 6. 87 (2H, d), 6. 80 (2H, d), 5. 13 (2H, s), 5. 01 (2H, s), 4. 13 (2H, s), 4. 02 (1H, t), 3. 51 (11H, m), 2. 72 (2H, m), 1. 79 (3H, s). <Embodiments 30>(S)- 3 - (4 - (4 - ((4 - (5 - isopropyl - 1, 2, 4 -Oxadiazole- 3 - yl) piperidinyl-DIN - 1 - yl) methyl) benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, 3 - isopropyl - 5 - (piperidine - 4 - yl) - 1, 2, 4 - oxadiazole using but, the embodiments 21 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 63 (2H, d), 7. 46 (2H, d), 7. 30 (2H, d), 6. 86 (2H, d), 5. 05 (2H, d), 4. 13 (2H, m), 4. 03 (1H, t), 3. 61 (1H, s), 3. 43 (2H, s), 3. 10 (1H, m), 2. 92 (4H, m), 2. 73 (2H, m), 2. 30 (2H, m), 1. 83 (3H, s), 1. 32 (6H, d). <Embodiments 31>(S)- 3 - (4 - (4 - ((4 - (5 - isopropyl - 1, 2, 4 -Oxadiazole- 3 - yl) piperazinylA - 1 - yl) methyl) benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, 3 - isopropyl - 5 - (piperazine - 1 - yl) - 1, 2, 4 - oxadiazole using but, the embodiments 21 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 61 (2H, d), 7. 49 (2H, d), 7. 30 (2H, d), 6. 87 (2H, d), 5. 05 (2H, s), 4. 15 (4H, m), 4. 02 (1H, t), 3. 49 (3H, m), 2. 81 (3H, m), 1. 83 (3H, s), 1. 24 (6H, d). <Embodiments 32>(S)- 3 - (4 - (4 - ((4 - (4 - (Methylsulfonyl)Phenyl) - 5, 6 -Dihydropyridine-1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, obtained in the manufacturing example 9 4 - (4 - (methylsulfonyl) phenyl) - 1, 2, 3, 6 - tetrahydropyridine hydrochloride using but, the embodiments 21 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, DMSO): δ 7. 95 (2H, d), 7. 75 (2H, d), 7. 63 (2H, d), 7. 44 (2H, d), 7. 30 (2H, d), 6. 98 (2H, d), 6. 37 (1H, s), 5. 14 (2H, s), 4. 45 (2H, t), 6. 97 (1H, s), 6. 82 (4H, m), 3. 27 (4H, s), 2. 84 (2H, s), 2. 59 (2H, d), 1. 77 (3H, s). <Embodiments 33>(S)- 3 - (4 - (4 - ((4 - (4 - (3 - (Methylsulfonyl)Propoxy)Phenyl) - 5, 6 -High diDraw pyridine -1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, obtained in the manufacturing example 11 4 - (4 - (3 - (methylsulfonyl) propoxy) phenyl) - 1, 2, 3, 6 - tetrahydropyridine hydrochloride using but, the embodiments 21 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 66 (2H, d), 7. 49 (2H, d), 7. 32 (2H, d), 7. 15 (2H, d), 6. 90 (2H, d), 6. 82 (2H, d), 5. 06 (2H, s), 4. 18 (2H, s), 4. 09 (3H, m), 3. 58 (2H, s), 3. 26 (2H, m), 2. 97 (3H, s), 2. 81 (5H, m), 2. 62 (3H, s), 2. 32 (2H, m), 1. 96 (2H, d), 1. 83 (3H, s). <Embodiments 34>(3S) - 3 - (4 - (4 - (1 - (3, 4 -Dihydroisoquinoline-2 (1H) - yl) ethyl)togetherqualitative jade city) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing (S)- ethyl 3 - (4 - (4 - ((methyl opinion gun Neel jade city) methyl) benzyloxy) phenyl) - 4 - inosinate [heyk[heyk] formation employed in place, said obtained in example 12 (3S) - ethyl 3 - (4 - (4 - (1 - bromoethyl) benzyloxy) phenyl) - 4 - inosinate [heyk[heyk] formation but using, the same embodiments 21 performs in a manner that the desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 12. 98 (1H, s), 7. 61 (6H, m), 7. 30 (4H, m), 6. 92 (2H, t), 5. 08 (2H, s), 4. 29 (2H, s), 4. 06 (1H, s), 3. 81 (1H, s), 3. 51 (2H, s), 3. 21 (2H, m), 2. 75 (2H, m), 1. 95 (2H, d), 1. 84 (3H, s). <Embodiments 35>(S)- 3 - (4 - (4 - ((4 - (4 -Hydroxyphenyl) Piperazine - 1 - yl)Methyl)Benzyl jadeIn) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, for example 4 - 10 obtained in the above (1, 2, 3, 6 - tetrahydropyridine - 4 - yl) phenol hydrochloride using but, the embodiments 21 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 8. 80 (1H, s), 7. 41 (2H, d), 735 (2H, d), 7. 28 (2H, d), 6. 94 (2H, d), 6. 74 (2H, d), 6. 63 (2H, d), 5. 06 (2H, s), 3. 94 (1H, t), 3. 62 (3H, s), 2. 95 (4H, s), 2. 61 (2H, d), 1. 77 (3H, s). <Embodiments 36>(S)- 3 - (4 - (4 - ((4 - (4 - (3 - (Methylsulfonyl)Propoxy)Phenyl) Piperazine - 1 -Yl) methyl) benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, 1 - (4 - (3 - (methylsulfonyl) propoxy) phenyl) piperazine hydrochloride using but, the embodiments 21 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 12. 32 (1H, s), 7. 42 (4H, m), 7. 29 (2H, d), 6. 96 (2H, d), 6. 83 (4H, q), 5. 06 (2H, s), 4. 02 (2H, t), 3. 92 (1H, t), 3. 52 (2H, s), 3. 25 (2H, t), 3. 01 (7H, m), 2. 61 (2H, d), 2. 09 (2H, m), 1. 77 (3H, d). <Embodiments 37>sodium (S)- 3 - (4 - (4 - (Isoindoline- 2 -Ylmethyl)Benzyloxy)Phenyl)[heyk[heyk]- 4 -Polycarbonate of inosinate Nitrogen atmosphere, 27 (S) made in 500 ml cap the embodiments - 3 - (4 - (4 - (- 2 - isoindoline ylmethyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate 0. 4 g and ethanol which can be after dissolving agitating, 3N sodium hydroxide aqueous solution 0. A 3 ml enemy-gate. Then, the end point of the reaction stirring at room temperature, then cream a reaction solution, filtering by adding isopropyl alcohol resulting solids desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 09 (2H, d), 7. 03 (2H, d), 6. 97 (2H, d), 6. 85 (2H, m), 6. 75 (2H, m), 6. 57 (2H, d), 4. 54 (2H, s), 3. 81 (1H, t), 3. 36 (4H, s), 3. 31 (2H, s), 2. 33 (2H, d), 1. 54 (3H, d). <Embodiments 38>L - lysine (S)- 3 - (4 - (4 - (Isoindoline- 2 -Ylmethyl)Benzyloxy)Phenyl)[...]Inosinate of gate - 4 - hydroxybenzoate Nitrogen atmosphere, cap the embodiments made in 27 (S)- 3 - (4 - (4 - (- 2 - isoindoline ylmethyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate 0. 4g and ethanol which can be after dissolving agitating, L - lysine 0. 12g some excellent. Then, to the reaction temperature 50 °C temperature 50 °C by stirring in 30 minutes, 30 minutes stirring the cooling back-gate. The end point of the reaction, resulting solids contained in a desired compound are obtained. 1H NMR (400MHz, D2O): δ 7. 03 (6H, s), 6. 83 (2H, s), 6. 74 (2H, s), 6. 54 (2H, s), 4. 53 (2H, s), 3. 77 (1H, s), 3. 54 (5H, m), 2. 88 (2H, t), 2. 28 (2H, s), 1. 74 (2H, m), 1. 62 (3H, m), 1. 42 (3H, s), 1. 35 (3H, m). <Embodiments 39>(S)- 3 - (4 - (4 - ((4 - (4 -Fluorophenyl) - 5, 6 -Dihydropyridine-1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, 4 - (4 - fluorophenyl) - 1, 2, 3, 6 - tetrahydropyridine hydrochloride using but, the embodiments 21 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 69 (2H, d), 7. 48 (2H, d), 7. 32 (4H, m), 7. 04 (2H, t), 6. 86 (2H, d), 5. 90 (1H, s), 5. 03 (2H, s), 4. 30 (2H, s), 4. 02 (1H, t), 3. 71 (2H, s), 3. 54 (2H, s), 3. 31 (2H, s), 2. 73 (2H, m), 1. 81 (3H, d). <Embodiments 40>(S)- 3 - (4 - (4 - ((4 - (4 -Methoxyphenyl) Piperazine - 1 - yl)Methyl)Benzyloxy)Phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, 4 - (4 - methoxyphenyl) - 1, 2, 3, 6 - tetrahydro pyridine using but, the embodiments 21 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 64 (2H, d), 7. 48 (2H, d), 7. 31 (2H, d), 6. 94 (2H, s), 6. 86 (4H, t), 5. 04 (2H, s), 4. 21 (2H, s), 4. 03 (1H, t), 3. 78 (3H, s), 3. 60 (2H, s), 3. 47 (2H, s), 3. 05 (2H, s), 2. 73 (2H, m), 1. 82 (3H, s). <Embodiments 41>sodium (S)- 3 - (4 - (4 - ((3, 4 -Dihydroquinoline-1 (2H) - yl)Methyl)BenzylOxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] of inosinate Step 1: (S)- 3 - (4 - (4 - ((3, 4 -Dihydroquinoline-1 (2H) - yl)Methyl)Benzyloxy)[...]Carbonyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, 1, 2, 3, 4 - tetrahydroquinolines using but, the embodiments 21 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 02 (2H, d), 6. 76 (2H, d), 6. 69 (2H, d), 6. 43 (4H, m), 6. 21 (1H, s), 6. 02 (1H, s), 4. 24 (2H, s), 3. 84 (3H, s), 2. 68 (2H, s), 2. 37 (2H, d), 2. 14 (2H, s), 1. 47 (3H, s), 1. 35 (2H, s). Step 2: sodium (S)- 3 - (4 - (4 - ((3, 4 -Dihydroquinoline-1 (2H) - yl)Methyl)Benzyl jadeIn) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] of inosinate (S)- 3 - (4 - (4 - (- 2 - isoindoline ylmethyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate instead, obtained in the above steps 1 (S)- 3 - (4 - (4 - ((3, 4 - dihydroquinoline -1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - but using wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate, the same embodiments 37 performs in a manner that the desired compound are obtained. 1 H NMR (400MHz, D2 O): δ 7. 01 (2H, d), 6. 74 (2H, d), 6. 68 (2H, d), 6. 42 (4H, m), 6. 15 (1H, s), 6. 02 (1H, s), 4. 25 (2H, s), 3. 79 (3H, s), 2. 62 (2H, s), 2. 34 (2H, d), 2. 12 (2H, s), 1. 45 (3H, s), 1. 32 (2H, s). <Embodiments 42>potassium (S)- 3 - (4 - (4 - ((3, 4 -Dihydroquinoline-1 (2H) - yl)Methyl)togetherqualitative jade city) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] of inosinate (S)- 3 - (4 - (4 - ((4 - (4 - fluorophenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate instead, obtained in step 1 the embodiments 41 (S)- 3 - (4 - (4 - ((3, 4 - dihydroquinoline -1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - but using wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate, the embodiments 25 performs in a manner identical to the desired compound are obtained. 1 H NMR (400MHz, D2 O): δ 6. 97 (2H, d), 6. 71 (2H, d), 6. 63 (2H, d), 6. 45 (2H. S), 6. 38 (2H, d), 6. 13 (1H, s), 5. 98 (1H, s), 4. 20 (2H, s), 3. 71 (3H, m), 2. 58 (2H, s), 2. 32 (2H, s), 2. 15 (2H, s), 1. 43 (3H, s), 1. 29 (2H, s). <Embodiments 43>(S)- 3 - (4 - (4 - ((4 - (Benzo thiazole- 2 - yl) piperazine - 1 - yl)Methyl)BenzylOxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, for example 13 2 - (piperazine - 1 - yl) benzo obtained in said thiazole hydrochloride using but, the embodiments 21 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, DMSO): δ 10. 87 (1H, s), 7. 85 (1H, d), 7. 55 (5H, m), 7. 31 (3H, m), 7. 14 (2H, t), 6. 96 (2H, d), 5. 13 (2H, s), 4. 40 (2H, s), 4. 17 (2H, d), 3. 95 (1H, t), 3. 57 (3H, t), 3. 22 (3H, s), 2. 57 (2H, d), 1. 78 (3H, d). <Embodiments 44>(S)- 3 - (4 - (4 - ((4 - (5 -Propyl pyrimidine- 2 - yl) piperazine - 1 - yl)Methyl)Benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, 2 - (piperazine - 1 - yl) - 5 - propyl pyrimidine hydrochloride obtained in the manufacturing example 14 but using, the same embodiments 21 performs in a manner that the desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 8. 20 (2H, s), 7. 62 (2H, d), 7. 47 (2H, d), 7. 30 (2H, d), 6. 85 (2H, d), 5. 08 (2H, s), 4. 80 (2H, d), 4. 17 (2H, s), 4. 03 (1H, t), 3. 84 (1H, t), 3. 43 (2H, s), 2. 74 (4H, m), 2. 43 (2H, t), 1. 83 (3H, d), 1. 59 (2H, q), 0. 94 (3H, t). <Embodiments 45>(S)- 3 - (4 - (4 - ((4 - (5 -Cyano roh blood [tin[tin]- 2 - yl) piperazine - 1 - yl)Methyl)togetherqualitative jade city) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, 6 - (piperazine - 1 - yl) nicotinate hydrochloride knight reelroh mote said obtained in example 15 but using, the same embodiments 21 performs in a manner that the desired compound are obtained. 1 H NMR (400MHz, DMSO): δ 11. 20 (1H, s), 8. 56 (1H, s), 7. 99 (1H, d), 7. 63 (1H, d), 7. 55 (1H, d), 7. 27 (2H, d), 7. 04 (1H, d), 6. 95 (2H, d), 5. 12 (2H, s), 4. 57 (2H, d), 4. 35 (2H, s), 3. 95 (1H, t), 3. 39 (5H, m), 2. 90 (2H, m), 2. 59 (2H, d), 1. 77 (3H, d). <Embodiments 46>(3S) - 3 - (4 - (4 - ((3 -Phenylpyrrolidine- 1 - yl)Methyl)Benzyloxy)Phenyl)[heyk[heyk]-4 - inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 1, 2, 3, 4 - tetrahydro isoquinoline instead to, but using 3 - phenyl blood Raleigh [tin[tin], the embodiments 21 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 12. 64 (1H, s), 7. 66 (2H, s), 7. 46 (2H, d), 7. 32 (7H, m), 6. 86 (2H, d), 5. 02 (2H, s), 4. 28 (2H, m), 4. 04 (1H, t), 3. 87 (2H, s), 3. 73 (1H, s), 3. 18 (1H, s), 2. 89 (1H, m), 2. 84 (3H, m), 2. 61 (1H, s), 2. 41 (1H, s), 2. 19 (1H, s), 1. 81 (3H, d). <Embodiments 47>sodium (S)- 3 - (4 - (4 - ((4 - (4 -Methoxyphenyl) Piperazine - 1 - yl)Methyl)BenzylOxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] of inosinate (S)- 3 - (4 - (4 - (- 2 - isoindoline ylmethyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate instead, obtained in the embodiments 40 (S)- 3 - (4 - (4 - ((4 - (4 - methoxyphenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - but using wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate, the same embodiments 37 performs in a manner that the desired compound are obtained. 1 H NMR (400MHz, MEOC): δ 7. 33 (2H, d), 7. 26 (1H, d), 7. 11 (1H, s), 6. 96 (8H, m), 5. 04 (2H, s), 4. 04 (1H, t), 3. 76 (3H, s), 3. 32 (4H, m), 3. 21 (4H, m), 2. 52 (2H, m), 1. 80 (3H, s). <Embodiments 48>(S)- 3 - (4 - (4 - (2 - (6 -Methoxy- 3, 4 -Dihydroisoquinoline-2 (1H) - one)Ethyl) benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing Step 1: ethyl (S)- 3 - (4 - (4 - (2 - (6 -Methoxy- 3, 4 -Dihydroisoquinoline-2 (1H) -Yl) ethyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] of inosinate Nitrogen atmosphere, 6 - methoxy - 1, 2, 3, 4 - tetrahydro isoquinoline 0 to cap. 5g agitating and injecting a DMF 20 ml after dissolving, purification of cesium 1 at room temperature. 1g been applying. 30 minutes, ethyl - 3 - (S) obtained in the manufacturing example 16 (4 - (4 - (2 - (methyl opinion gun Neel jade city) ethyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate 1. 0g the enemy it inflicted behind a, 12 at room temperature stirring time-gate. The reaction terminates in distilled water (brine) then ethyl acetate the enemy it inflicted evaporants knife of magnesium sulfate anhydride is carried out by extracting wash after drying was and concentrated. After this silica column chromatography separation desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 35 (2H, d), 7. 30 (2H, d), 7. 23 (2H, d), 7. 00 (1H, d), 6. 85 (2H, d), 6. 80 (1H, d), 6. 70 (1H, d), 5. 00 (2H, s), 4. 30 (2H, m), 4. 13 (2H, m) 4. 03 (1H, t), 3. 80 (3H, s), 3. 58 (6H, m), 3. 30 (2H, s), 2. 78 (2H, m), 1. 86 (3H, d), 1. 28 (3H, m). Step 2: (S)- 3 - (4 - (4 - (2 - (6 -Methoxy- 3, 4 -Dihydroisoquinoline-2 (1H) - yl) toCefuroxime) benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate [eyk[eyk] Hour [tu[tu] of wing Nitrogen atmosphere, step 1 ethyl - 3 - (S) made in the cap (4 - (4 - (2 - (6 - methoxy - 3, 4 - dihydroisoquinoline -2 (1H) - yl) ethyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate 0. 5g a THF, methanol and distilled water is charged into a agitating after dissolving, 0 lithium hydroxide at room temperature. 5g 1 hours stirring slowly adding a-gate. The reaction terminates, a 1M aqueous hydrochloric acid solution to pH 2 to 3 and the extracted using ethyl acetate using an acidic and reduced pressure drying target compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 7. 35 (2H, d), 7. 30 (2H, d), 7. 23 (2H, d), 7. 00 (1H, d), 6. 85 (2H, d), 6. 80 (1H, d), 6. 70 (1H, d), 5. 00 (2H, s), 4. 30 (2H, m), 4. 03 (1H, t), 3. 80 (3H, s), 3. 58 (6H, m), 3. 30 (2H, s), 2. 78 (2H, m), 1. 86 (3H, d). <Embodiments 49>(S)- 3 - (4 - (4 - (2 - (Isoindoline- 2 - yl) ethyl)Benzyloxy)Phenyl)[heyk[heyk]- 4 -Inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 6 - methoxy - 1, 2, 3, 4 - tetrahydro isoquinoline instead to, isoindoline using but, the embodiments 48 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, CDCl3 ): Δ 13. 57 (1H, s), 7. 38 (3H, m), 7. 29 (7H, m), 6. 90 (2H, d), 5. 03 (4H, m), 4. 28 (2H, s), 4. 08 (1H, t), 3. 48 (2H, m), 3. 34 (2H, m), 2. 80 (2H, m), 1. 83 (3H, d). <Embodiments 50>(S)- 3 - (4 - (4 - (2 - (3, 4 -Dihydroisoquinoline-2 (1H) - yl) ethyl)BenzylOxy) phenyl) - 4 - [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu] manufacturing 6 - methoxy - 1, 2, 3, 4 - tetrahydro isoquinoline instead to, 1, 2, 3, 4 - tetrahydro isoquinoline using but, the embodiments 48 performs in a manner equal to desired compound are obtained. 1 H NMR (400MHz, DMSO): δ 7. 44 (2H, d), 7. 38 (2H, d), 7. 27 (5H, m), 7. 22 (1H, d), 6. 94 (2H, d), 5. 07 (2H, s), 4. 64 (1H, d), 4. 38 (1H, s), 3. 95 (1H, t), 3. 77 (1H, s), 3. 39 (2H, s), 3. 16 (4H, m), 2. 26 (2H, d), 1. 77 (3H, d), 1. 84 (3H, d), 1. 29 (3H, t). <Embodiments 51>sodium (S)- 3 - (4 - (4 - ((6 -Methoxy- 3, 4 -Dihydroisoquinoline-2 (1H) -Yl) methyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] of inosinate (S)- 3 - (4 - (4 - (- 2 - isoindoline ylmethyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate instead, obtained in the embodiments 25 (S)- 3 - (4 - (4 - ((6 - methoxy - 3, 4 - dihydroisoquinoline -2 (1H) - yl) methyl) benzyloxy) phenyl) - 4 - but using wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate, the same embodiments 37 performs in a manner that the desired compound are obtained. 1 H NMR (400MHz, D32 O): δ 7. 10 (2H, d), 7. 02 (2H, d), 6. 95 (2H, d), 6. 55 (2H, d), 6. 40 (1H, d), 6. 34 (2H, s), 4. 53 (2H, s), 3. 83 (1H, t), 3. 39 (3H, s), 3. 17 (2H, s), 3. 05 (2H, s), 2. 37 (4H, m), 2. 20 (2H, s), 1. 57 (3H, s). <Comparison example 1>[(3 [(3 <Comparison example 2>(3S) - 3 - (4 - {[4 - (1' H - spiro [Indene- 1, 4 '- piperidine] -1' -Ylmethyl) TogetherBe] oxy} phenyl) - 4 - [heyk[heyk]one Roh wing [eyk[eyk] Hour [tu[tu] manufacturing (3S) - 3 - (4 - {[4 - (1 'H - spiro [indene - 1, 4' - piperidine] -1 ' - ylmethyl) benzyl] oxy} phenyl) - 4 - International call public Patent WO2011/046851 [heyk[heyk]one Roh wing [eyk[eyk] Hour [tu[tu] prepared with known methods. <Comparison example 3>4 - (3 -lung stipend city it cuts the qualitative amino)Phenyl pro blood Roh [ik[ik][eyk[eyk] Hour [tu[tu] Manufacturing 4 - (3 - lung stipend city it cuts the qualitative amino) phenyl pro blood Roh [ik[ik][eyk[eyk] Hour [tu[tu] prepared with known methods. The chemical structure of the compounds prepared in the embodiments 1 to table 1 - 51 to organize his shown. <Experiment example 1>3 - (4 - (Benzyloxy)Phenyl)[heyk[heyk]- 4 -Inosinate wing By acid derivatives GPR40 stageActive hundred questions degree evaluation The novel 3 - (4 - (benzyloxy) phenyl) - 4 - GPR40 [heyk[heyk] inosinate wing acid derivatives such as activation of which code for assessing for conducting experiments. GPR40 protein activity due to intracellular calcium changes in concentration through a novel 3 - (4 - (benzyloxy) phenyl) - 4 - GPR40 [heyk[heyk] inosinate wing acid derivatives was assessed by obtaining the protein. First, HEK-a 293 cells using Fugene HD (promega, E2311) (Origene, RC218370) human GPR40 DNA was transformed. 96 - well black clear bottom plate (Costar, 3603) HEK-a 293 cells transformed to him as which is included. 24 hours, removed from cell culture (Fetal Bovine Serum, FBS) added with 1% small [thay[thay]oh serumit puts the hemp cloth noseeagle modified medium (Dulbecco's Modified Eagle Medium, DMEM, 50ul) was injected into the gate. Calcium concentration measurement reagent to each well of cells 50 μL Fluo provided 4 (enhancing with [thu[thu] [keyn[keyn], F10471) administration, 37 °C incubator in him as time 2. Example compound 4 - 1, 2 during culturing of 20 mm compared embodiments compounds (2 - hydroxyethyl) - 1 - piperidinyl the position ethane opinion phone it buys buffer solution (4 - (2 a-hydroxyethyl) - 1 a-piperazineethanesulfonic acid, HEPES) added with 1 × HBSS (Hank's Buffered Salt Solution) diluting a sample processing cells to work through. 2 culture start time has elapsed (Molecular Devices) device for positioning the sieves 3 cis prepared sample (Flexstation 3) flex yarn station and to automatically injected into cells, SoftMax? Names of 120 seconds Pro intracellular calcium changes in concentration were measured. The, die as a nothing control armymethyl opinion bombing death id (DMSO) injected into cells to calcium changes in concentration were measured. Calcium concentration results from expressions using measured to 1 when the degree of the GPR40 protein activity, sample therefrom by GPR40 active EC50 His values are derived. For table 2 have shown to result. In the table 2, A grade 0. Less than 20 μm; B grade 0. 20 - 0. 30 μm and; 0 * C ratings. Greater than 30 μm. The table as shown in the variation 2, the embodiments in accordance with the compounds are active at low concentrations is superior than that of the GPR40 protein can be. In particular, the embodiments 7, 9, 11, 12, 14, 27, 28, 37 and 38 0. 20 μm or less activate GPR40 extremely low concentration 50% protein, Ca within cells2+ In order to increase the ability to this comparison example 1 (B, 0. 28 μm) than his car to 1:1 by weight. Thus, according to the invention novel 3 - (4 - (benzyloxy) phenyl) - 4 - GPR40 protein activity rate [heyk[heyk] inosinate wing acid derivative is superior, to conventional GPR40 antidiabetic (comparison example 1) known to promote saving insulin protein activity compared to similar or improved GPR40 protein activity ratio rotated, pharmaceutical composition derived obesity, diabetes of type I, diabetes of type II, coincidental glucagon-like peptide, insulin resistance, hyperglycemia, aims, high the Holy Land bloodletting symptoms, hypercholesterolemia or mixed dyslipidemia, hypertriglyceridemia, such as sick X can be useful pharmaceutical compositions for preventing or treating metabolic disorders. *<Experiment example 2>calcium flux (Calcium Flux) analysis The novel 3 - (4 - (benzyloxy) phenyl) - 4 - GPR40 [heyk[heyk] inosinate wing acid derivatives activated GPCR assay for evaluating calcium flow rate according to engine millimeter can microcontroller (millipore) to professional through a commercial implementation. DMSO (Dimethyl sulfoxide), PBS (Phosphate buffered saline), DW (Distilled Water) obtained by dissolving a compound such as EMD millipore's GPCR profiler analysis buffer 3 times concentration (profiler assay buffer) embodiments to work through. Similarly, nothing control group (vehicle) and positive controls (comparison example 1 and 3) was used to confirm the accuracy of analysis. EMD millipore's GPCR profiler analysis buffer was prepared using all well. EMD millipore's GPCR profiler analysis buffer is 20 mm HEPES (4 - (2 a-hydroxyethyl) - 1 a-piperazineethanesulfonic acid) 2 on. 5 mm Probenecid ((dipropylsulfamoyl) 4 - benzoic acid) which, pH 7. 4 adjusted to HBSS (Hanks Balanced salt solution) are disclosed. Embodiments compounds (duplicate) reproducing to each concentration was used. Positive controls (comparison example 1 and 3) for each GPCR (G protein a-coupled receptors) as to the nothing control group (Vehicle) was prepared. For each GPCR positive controls (comparison example 1 and 3) E concentrations to the highest activityMax Was included. Enhancer analysis the FLIPR (Agonist assay)TETRA Machine performed using a transparent conductive layer, a light emitting reference value measured fluorescence (fluorescence) (luminescence baseline) embodiments compounds, nothing control group and positive controls (comparison example 1 and 3) was added to analyzing plate. Embodiments compounds for activity, conducting GPCR activity analysis 180 seconds. The reference value is subtracted fluorescence values of positive controls (comparison example 1 and 3) EMax Compared to nothing control army, activity was calculated as a percentage in the first to fourth. Also data with nothing control army EC50 Value for evaluating quality of each plate percentage inhibition percentage was calculated statistical value indicating active between repeated data values. Analysis data is suitable as the additional experiments is not conducting. All concentration dependent the graph was prepared by using GraphPad Prism. The graph includes a Sigmoidal dose response was modified by fixing the minimum 0, better to expected values for maximum efficacy was 100 is secured. Also 1 and 3 have shown to result bright. Figure 1 shows a concentration of compounds 9 and comparison example 1, 3 also embodiments according to positive measuring graph GPR40 activation protein are disclosed. As shown in fig. 1, embodiments 1 and 3 to the activity of compounds this comparison example GPR40 than by the exceptionally low concentration necessary to reach 50% (1 nm less than that measurable concentration) been can be ascertained. Specifically, as shown in the table 3, the example embodiments in accordance with the comparison compound 1 (14 nm) and 3 (27 nm) significantly lower than concentrations in the activating GPR40 ETEC. Thus, according to the invention novel 3 - (4 - (benzyloxy) phenyl) - 4 - GPR40 protein activity rate [heyk[heyk] inosinate wing acid derivative is superior, to conventional active protein to GPR40 (comparison example 1 and 3) known to promote saving insulin also shown on GPR40 operates protein activity rate is dramatically and low, pharmaceutical composition derived obesity, diabetes of type I, diabetes of type II, coincidental glucagon-like peptide, insulin resistance, hyperglycemia, aims, high the Holy Land bloodletting symptoms, hypercholesterolemia or mixed dyslipidemia, hypertriglyceridemia, such as sick X can be useful pharmaceutical compositions for preventing or treating metabolic disorders. <Experiment example 3> CYP Inhibiting analysis The novel 3 - (4 - (benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate drug interaction between wing acid derivatives such as conducting experiments to evaluate. As drug metabolism CYP enzyme is an enzyme involved, this enzyme inhibitory effects on the drug dose and according with other drugs due to the administration of a combination process from prediction of toxicity rehmanniae concentration. The, embodiments in accordance with the present invention using compounds that are present in human CYP3A 4, CYP2C 9, CYP1A 2, CYP2D 6 and CYP2C 19 determined the inhibitory effects. The, inhibiting kit (P2862) enhancing a CYP 2D 6 with [thu[thu] [keyn[keyn] deflection, CYP1A 2, CYP2C 9, CYP2C 19, CYP3A 4 BD GENTEST inhibiting kit (459100, 459300, 459400, 459500) as well as a. In the case of enhancing the with [thu[thu] [keyn[keyn] kit, final test substance concentration of experiment 2. Preparation of diluted in a distilled water to be 5 × his. The first and enhancing the P450 BACULOSOMES with [thu[thu] [keyn[keyn] kit? Reagents with the reproducing apparatus (100X) Vivid a? CYP450 reaction buffer solution (2 ×) CYP450 concentrations to a focus error signal is thus diluted with the Royal. U a-bottom 96 - well plate to prepare a 2. 5 × sample material (80 μL) diluted with P450 BACULOSOMES? Reagent mixture (100 μL) after 20 minutes before him as - main pressure is lowered. Vivid? CYP450 NADP + with the substrate (100 ×) substrate with the aid of a Vivid CYP450 concentration on the kind? CYP450 reaction buffer solution (2 ×) diluted in a preparation of him. After the end (Nicotinamide adenine dinucleotide phosphate) - NADP clinically pre-substrate solution (20 μL) into the base 1 with respect to the reaction time. In detecting the fluorescence wavelength affected by substances while using reactants in microplate reader white plate finished reading (of woman wavelength CYP 2D 6:400 nm, absorption wavelength: 502 nm). In the case of test substance concentration of 50 × BD GENTEST kit preparation of diluted in a final experiment so knight reel process for him. Coenzyme NADPH - distilled water mixture in its kit to coenzyme, G6PDH, recombinant vaccines for concentration presented in kit is thus diluted with the Royal. 50X test substance (4 μL) coenzyme NADPH - and mixture (96 μL) U-a bottom 96 - well plate in 10 minutes after mixing is carried out in a pre-amount-gate 37 °C incubator. The kit enzyme/substrate mixture in its buffer solution (0. 5M potassium phosphate (pH7. 4)), each CYP450 enzyme/substrate mixture was diluted in a predetermined concentration depends on the kind of CYP450 preparation of distilled water. 100 μL enzyme/substrate mixture is added 15 minutes into the base end plate pre-clinically (CYP 1A 2), 30 minutes or 1 time 30 minutes (CYP 2C 9) 37 °C (CYP 3A 4 and CYP 2C 19) during reaction with respect to the culture. The reactants in detecting the fluorescence wavelength affected by substances in finished reading white plate microplate reader (CYP 1A 2 and CYP 2C 19 woman wavelength: 410 nm, absorption wavelength: 460 nm; CYP 2C 9, and CYP 3A 4 woman wavelength: 409 nm, absorption wavelength: 530 nm). The percentage inhibition of rate when the measured values are nothing control group contrast sample material terms, the result shown in table 4. As appears in the table 4, the embodiments in accordance with the compounds are CYP 450 for low inhibitory activity, drug side effect occurs due to the interaction between risk low been can be ascertained. More specifically, according to the invention substantially in most compounds are CYP 1A 2, CYP 2C 9, CYP 2C 19, enzyme inhibitory rate of less than about 50% enzyme CYP 2D 6 and CYP 3A 4 mistletoe. In particular, conventional insulin secretion by enzyme CYP 2C 9 GPR40 clause glycosuria zero comparison example 1 promotes active protein used compounds of (81. 2%) compared to, embodiments compounds are characterized by relatively very low enzyme inhibitory activity mistletoe. Also, compared against enzyme CYP 2D 6 compounds of example 2 (63. 2%) compared to, embodiments compounds are characterized by relatively very low enzyme inhibitory activity mistletoe. Thus, according to the invention novel 3 - (4 - (benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing acid derivative is CYP enzyme inhibitory activity is dramatically and thus, by a combination with administration thereof can be applied to other drug containing pharmaceutical compositions unexpectedly give rise, diabetes type I, diabetes type II, coincidental glucagon-like peptide, insulin resistance, hyperglycemia, aims, high the Holy Land bloodletting symptoms, hypercholesterolemia or mixed dyslipidemia, hypertriglyceridemia, useful in the treatment of metabolic diseases including complications such as sick X can be used. <Experiment example 4>load per oral (Oral Glucose Tolerance Test; OGTT) 1 The novel 3 - (4 - (benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing acid derivatives represented by the formula which code for evaluating in vivo such as conducting experiments. 8 - 10 for detecting BAX (Sprague Dawley rat) between minimum 7 male sprag - week zerous who are species to an individual by using only the finger of time are provided to health after per oral (OGTT test) load imposed on wine. 16 to 18 to a nanoparticulate military North Korean Workers Party by separating each time after 5 mary randomly group embodiments 2, 3, 4, 6, 9, 12, 14, 16, 25, 29, 36, 37, 41, 43 and 44 respectively 10 mg/kg compounds that are produced in oral dose was administered. The, 5% polyethylene glycol/5% to twin 80/0 nothing control group (Vehicle). 25% car diplopia methyl cellulose (CarboxyMethyl Celluluse, CMC) solution (PEG 400/Tween 80/0. 25% CMC, 5% / 5% / 90%, v/v/v) comparable to oral administered. Sample 30 minutes long-term glucose administration (4 g/kg) was administered orally in an amount of 5 ml/kg. Then, the blood glucose oh queue - check active strip (Accu-a chek active strip, Roche diagnostic co.) for measuring a transparent conductive layer, the time required to measure the glucose administration reference -30 minutes, 0 minutes, 20 minutes, 40 minutes, and at least 60 120 minutes after a puncture pulse measured blood glucose AUC (Reduction ratio of AUC (%)) crude have strong rate to table 5 have shown to produce results. As shown in the 5 the table, the compounds are nothing control embodiments in accordance with the group-average 21. 9% has the effect than that of a method of lowering blood glucose can be effective in vivo. More specifically, in the case of known conventional stabilizer 16 GPR40 protein activity comparison example 1. 2% effect of lowering blood but the number of passes is identified, the embodiments in accordance with the compound is represented by the formula using rice flour is ETEC. In particular, in the case of 9, 12, 14, 29 and 37 embodiments each 24. 7%, 31. 0%, 27. 7%, 27. 1% and 28. 5% by comparison example 1 excellent agent in a method of lowering blood glucose agent in a contrast as in the picomolar. Thus, according to the invention novel 3 - (4 - (benzyloxy) phenyl) - 4 - GPR40 [heyk[heyk] inosinate wing acid derivative is cooled thereby excellent active protein in insulin-secreting effect represented by the formula containing pharmaceutical compositions unexpectedly give rise significantly and low heat curable obesity, diabetes of type I, diabetes type II, use my party, treating insulin resistance, hyperglycemia, aims, high the Holy Land bloodletting symptoms, hypercholesterolemia or mixed dyslipidemia, hypertriglyceridemia, useful pharmaceutical compositions for the treatment of metabolic disorders such as sick X can be. <Experiment example 5>load per oral (Oral Glucose Tolerance Test; OGTT) 2 The novel 3 - (4 - (benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing acid derivatives represented by the formula which code for evaluating in vivo such as conducting experiments. 22 - 23 (GK) for detecting BAX week zero obesity type II diabetes of type not minimize the finger of time are provided between which is a model male Goto a-Kakizaki 7 by using only load to an individual to health after per oral (OGTT test) implementation. 16 to 18 by separating each time after a nanoparticulate military North Korean Workers Party embryo randomly group 5, 5, 9, 14, 28, 37 and 47 embodiments compounds that are produced in each 0. 3 - 10 mg/kg oral dose was administered. The, 5% polyethylene glycol/5% to twin 80/0 nothing control group (Vehicle). 25% car diplopia methyl cellulose (CarboxyMethyl Celluluse, CMC) solution (PEG 400/Tween 80/0. 25% CMC, 5% / 5% / 90%, v/v/v) comparable to oral administered. Methyl cellulose (CarboxyMethyl Celluluse, CMC) solution (PEG 400/Tween 80/0. 25% CMC, 5% / 5% / 90%, v/v/v) comparable to oral administered. Sample 60 minutes long-term glucose administration (4 g/kg) was administered orally in an amount of 5 ml/kg. Then, the blood glucose oh queue - check active strip (Accu-a chek active strip, Roche diagnostic co.) for measuring a transparent conductive layer, the time required to measure glucose administration reference - 60, 0, 20, 40, 60 and 120 minutes after a puncture pulse measured blood glucose AUC (Reduction ratio of AUC (%)) crude have strong rate to table 6 have shown to produce results. In the table 6, 35 A ratings. Is greater than 0%; 25 B ratings. 0 - 35. 0% and; C grade 25. Less than 0%. As shown in the table 6, the same dose compared to the example embodiments in accordance with the compound 1 (10 mg/kg) group nothing control in average 30. 0% or more hypoglycemic effect her. More specifically, in the case of comparison example 1 10 mg/kg at doses 25. (B) 3% but agent in a method of lowering blood glucose, for example in the case of 3 mg/kg administration group compared in embodiments 1 5, 9, 14, 28, 37 and 47 of a frame hypoglycemic efficacy and viscoelasticity. In particular, an address group 35 37 9 and the same 10 mg/kg administration embodiments respectively. 0% or more by specifying the Manchester carry glucose, compared to comparison example 1 very good agent in a ETEC. Thus, according to the invention novel 3 - (4 - (benzyloxy) phenyl) - 4 - GPR40 [heyk[heyk] inosinate wing acid derivative is cooled thereby excellent active protein in insulin-secreting effect represented by the formula containing pharmaceutical compositions unexpectedly give rise significantly and low heat curable obesity, diabetes of type I, diabetes type II, coincidental glucagon-like peptide, insulin resistance, hyperglycemia, aims, high the Holy Land bloodletting symptoms, hypercholesterolemia or mixed dyslipidemia, hypertriglyceridemia, useful pharmaceutical compositions for the treatment of metabolic disorders such as sick X can be. <Experiment example 6>load per oral (Oral Glucose Tolerance Test; OGTT) 3 The novel 3 - (4 - (benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing acid derivatives represented by the formula which code for evaluating in vivo such as conducting experiments. 29 - 30 week zero obesity type II diabetes mellitus (Otsuka Long non-Evans Tokushima fatty) 7 which is a model male finger of time are provided to health after detecting BAX OLETF between minimum load per oral (OGTT test) to an individual by using only implementation. 16 - 18 5 5, 9, 14, 28, 37 and 47 by separating each time after a nanoparticulate military North Korean Workers Party embryo randomly group embodiments compounds that are produced in each 1 - 10 mg/kg oral dose was administered. The, 5% polyethylene glycol/5% to twin 80/0 nothing control group (Vehicle). 25% car diplopia methyl cellulose (CarboxyMethyl Celluluse, CMC) solution (PEG 400/Tween 80/0. 25% CMC, 5% / 5% / 90%, v/v/v) comparable to oral administered. Sample 60 minutes long-term glucose administration (4 g/kg) was administered orally in an amount of 5 ml/kg. Then, the blood glucose oh queue - check active strip (Accu-a chek active strip, Roche diagnostic co.) for measuring a transparent conductive layer, the time required to measure glucose administration reference - 60, 0, 20, 40, 60 and 120 minutes after a puncture pulse measured blood glucose AUC (Reduction ratio of AUC (%)) crude have strong rate to table 7 have shown to produce results. In the table 7, 35 A ratings. Is greater than 0%; 25 B ratings. 0 - 35. 0% and; C grade 25. 0 less than. As shown in the table 7, the same dose compared to the example embodiments in accordance with the compound 1 (10 mg/kg) in average 35 nothing control group. 0% or more hypoglycemic effect her. More specifically, in the case of 10 mg/kg 31 in comparison example 1. (B) 6% but agent in a method of lowering blood glucose, 1 mg/kg administration group compared in the case of example 1 embodiments 9 and 37 better hypoglycemic efficacy and viscoelasticity. In particular, 10 mg/kg administration group 9 and 37 35 such as a capacity embodiments respectively. 0% or more by specifying the Manchester carry glucose, compared to comparison example 1 very good agent in a ETEC. Thus, according to the invention novel 3 - (4 - (benzyloxy) phenyl) - 4 - GPR40 [heyk[heyk] inosinate wing acid derivative is cooled thereby excellent active protein in insulin-secreting effect represented by the formula containing pharmaceutical compositions unexpectedly give rise significantly and low heat curable obesity, diabetes of type I, diabetes type II, coincidental glucagon-like peptide, insulin resistance, hyperglycemia, aims, high the Holy Land bloodletting symptoms, hypercholesterolemia or mixed dyslipidemia, hypertriglyceridemia, useful pharmaceutical compositions for the treatment of metabolic disorders such as sick X can be. <Experiment example 7>blood oral administration GLP-a 1 (Glucagon- Like peptide-a 1) increasing the concentration Measuring The novel 3 - (4 - (benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate oral administration of GLP-a 1 wing acid derivatives such as assess blood concentration curve for conducting experiments. 10 - 12 male SD (Sprague Dawley rat) 7 for detecting BAX week zero minimum between individual by using only the finger of time are provided to health after conducting experiments. 16 - 18 to a nanoparticulate military North Korean Workers Party 5 by separating each time after each 10 - 100 mg/kg in embodiments 9 embryo randomly group (solvent administration volume: 5 ml/kg) was administered orally to capacity compounds that are produced. The, 5% polyethylene glycol/5% to twin 80/0 nothing control group (Vehicle). 25% car diplopia methyl cellulose (CarboxyMethyl Celluluse, CMC) solution (PEG 400/Tween 80/0. 25% CMC, 5% / 5% / 90%, v/v/v) comparable to oral administered. 20 minutes, heart scan about 0 direct blood collection through a whole blood sample. 5 ml blood collection and, EDTA (ethylenediaminetetraacetic acid) (Dipeptidyl peptidase-a 4) blood blood immediately DPP-a 4 inhibitors are included in the ice container is the treated sample tube was cooling of the container. The blood is 3600 rpm 10 minutes in separating blood plasma via centrifuging blood, ELISA kit (Millipore, USA) for measuring plasma GLP-a 1 through the separated blood plasma concentration of GLP-a 1 were measured. Also shown to result 2. Figure 2 shows a SD (Sprague Dawley rat) compounds and comparison example 1 to 9 also for detecting BAX embodiments oral-compounds, blood GLP-a 1 indicating graph livestock products are disclosed. Also as shown in the variation 2, compared to a comparison example 1 compound is glucose treatment group (Veh.), blood insulin secretion after administration promotes synergistic effect as a carbon concentration of hormone GLP-a 1 but, embodiments 9 compounds are administered at a predetermined concentration for detecting BAX SD increase blood concentration of GLP-a 1 as signal peptides. Thus, according to the invention novel 3 - (4 - (benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing acid derivatives compared to comparison example 1 GLP-a 1 promotion of the appearance of the skin and promoting the production and secretion, particularly conditions a very good efficacy in animal models as in the picomolar. Also, the degradation of beta cells by promoting secretion of GLP-a 1 weight increase can be treatable with the possibility of preventing expect obesity, diabetes of type I, diabetes type II, coincidental glucagon-like peptide, insulin resistance, hyperglycemia, aims, high the Holy Land bloodletting symptoms, hypercholesterolemia or mixed dyslipidemia, hypertriglyceridemia or useful pharmaceutical compositions for the treatment of metabolic disorders such as sick X can be. On the other hand, the compounds represented by formula 1 according to the present invention permits formulated into various types depending on the objective. The deaerator removes bubbles according to the present invention represented by formula 1 containing the compound as active ingredient formulated to exemplify the present invention limited to some method that are not correct. Example IV (Dipeptidyl peptidase-a 4, DPPIV) inhibitors with 8>d [pheyp[pheyp] mote [til[til] mote day [cu[cu]<experiment By combined administration (Oral Glucose Tolerance Test, OGTT) load per oral The novel 3 - (4 - (benzyloxy) phenyl) - 4 - IV (Dipeptidyl peptidase-a 4, DPPIV) d [pheyp[pheyp] mote [til[til] mote day [cu[cu][heyk[heyk] inosinate wing acid derivatives represented by the formula which code for evaluating blood glucose in vivo administration of curative such as conducting experiments. 8 - 1. Mouse (mouse) model experiment 29 to 30 male week zero dietary obesity model after 16 - 18 to a nanoparticulate military North Korean Workers Party mouse embryo (Diet a-Induced Obesity, DIO) 5 each time by separating each 30 - 100 mg/kg in embodiments 9 randomly group (solvent administration volume: 5 ml/kg) was administered orally to capacity compounds that are produced. The, the nothing control army 0. 5%, a comparable car diplopia methyl cellulose (CarboxyMethyl Celluluse, CMC) was administered orally. Also, d [pheyp[pheyp] mote [til[til] mote day [cu[cu] IV (Dipeptidyl peptidase-a 4, DPPIV) inhibitors (Sitagliptin) 10 mg/kg was administered a drug at the time of hit writing [lip[lip] alone is well known. Further, 10 mg/kg and embodiments 9 compounds that are produced in the combination (Sitagliptin) at the time of hit writing [lip[lip] 30 - 100 mg/kg was administered. The 0. 5%, 5 ml/kg was administered orally to car diplopia methyl cellulose (CarboxyMethyl Celluluse, CMC) and test materials. 60 minutes, glucose (4 g/kg) was administered orally in an amount of 5 ml/kg. Blood glucose is measured using oh [khwu[khwu] - check active strip (Accu-a chek active stript, Roche diagnostic co.) a transparent conductive layer, the time required to measure glucose administration reference - 60, 0, 20, 40, 60 and 120 have strong rate measured blood glucose AUC puncture pulse crude component (Reduction ratio of AUC (%)) 3 to 8 bright and also produce results have shown. As also shown in the table 3 and 8, at the time of hit writing [lip[lip] (10 mg/kg) alone not to induce embodiments using compounds that are produced in 9 (30 mg/kg, or 100 mg/kg) administered in combination than as in the picomolar excellent hypoglycemic effect. 8 - 2. SD (Sprague Dawley) Detecting BAX(Rat) model experiment 8 - 10 male SD (Sprague Dawley) 7 for detecting BAX week zero minimum using the finger of time are provided to health after OGTT test between individual by using only implementation. 16 - 18 to a nanoparticulate military North Korean Workers Party embryo after one time after the separation of the randomly group 6, vehicle (Vehicle) (0. 5%, carboxymethylcellulose (CarboxyMethyl Celluluse, CMC)) or 9 embodiments compounds of the 3 mg/kg, 1, 3, 10 mg/kg dose (Linagliptin) have writing [lip[lip] contamination in the administration, the administration of a combination embodiments compounds of group 9 (Linagliptin) 1, 3, 10 mg/kg 3 mg/kg capacity writing [lip[lip] contamination in each capacitance combination was administered. The compounds of the 9 to 10 ml/kg was administered oral expense it will grow with embodiments. Vehicle or compounds of embodiments 9 30 minutes after glucose administration (4 g/kg) was administered orally in an amount of 5 ml/kg. The Accu-a chek active strip (Roche diagnostic co.) for measuring when the blood glucose, glucose administration measuring time is measured reference - 30, 0, 20, 40, 60 and 120 have puncture pulse crude component, Blood glucose AUC (Reduction ratio of AUC (%)) have shown strong rate to produce results. Also shown is a table 9 and to AUC drop rate 7. (Mean ± SE) is precursor to experimental characteristic average standard error, differences between the band group regulated significance assays can 'GraphPad Prism 4' software (Graphpad co. , La Jolla, CA, USA) through a ring of one a-way ANOVA (a Dunnett method), p<0. 05 statistically significant difference when the number of passes is runner out. 9 compound when the administration of a combination of the drug at the writing [lip[lip] with contamination in embodiments, environmentally experiment may be observed and source of the AUC than have strong near value sources, each test group in vehicle about 5. 8 - 24. AUC (area under curve) showed strong effect of 4%. This compound when the administration of a combination drug-IV inhibitors d [pheyp[pheyp] mote [til[til] mote day [cu[cu] 9 embodiments, d [pheyp[pheyp] mote [til[til] mote day [cu[cu] IV inhibitors based drug efficacy can be formed in the amount of interrupt by a goniophotometer. Thus, according to the invention novel 3 - (4 - (benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing acid derivative is d [pheyp[pheyp] mote [til[til] mote day [cu[cu] IV (Dipeptidyl peptidase-a 4, DPPIV) inhibitors in combination with rehmanniae class of drugs, the drug are better hypoglycemic effect when administered alone, the derivatives and other active ingredient pharmaceutical composition comprises obesity, diabetes of type I, diabetes type II, use my party, treating insulin resistance, hyperglycemia, aims, high the Holy Land bloodletting symptoms, hypercholesterolemia or mixed dyslipidemia, hypertriglyceridemia, such as sick X can be useful in the prevention or treatment of metabolic disorders. <Experiment example 9>sulfonylureas (Sulfonylurea) Based medicaments by combined administration mirrorLoad per nine (Oral Glucose Tolerance Test, OGTT) The novel 3 - (4 - (benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing acid derivatives and sulfonylureas (Sulfonylurea) administration of a combination drug sequence represented by the formula which code for evaluating the blood glucose in vivo when such as conducting experiments. 29 to 30 male week zero dietary obesity model after 16 - 18 to a nanoparticulate military North Korean Workers Party mouse embryo (Diet a-Induced Obesity, DIO) 5 each time by separating compounds that are produced in embodiments 9 randomly group (solvent administration volume: 10 ml/kg) was administered orally to each 10 - 100 mg/kg capacity. The, the nothing control army 0. 5%, a comparable car diplopia methyl cellulose (CarboxyMethyl Celluluse, CMC) was administered orally. Also, sulfonylureas (Sulfonylurea) 10 mg/kg was administered a drug family is well known glimepiride (Glimepiride) alone. Further, glimepiride (Glimepiride) 10 mg/kg and embodiments 9 compounds that are produced in the combination 10 - 100 mg/kg was administered. The salt water (saline) and test materials to 5 ml/kg was administered orally. 60 minutes, glucose (4 g/kg) was administered orally in an amount of 5 ml/kg. Blood glucose is measured using oh [khwu[khwu] - check active strip (Accu-a chek active stript, Roche diagnostic co.) a transparent conductive layer, the time required to measure glucose administration reference - 60, 0, 20, 40, 60 and 120 have strong rate measured blood glucose AUC puncture pulse crude component (Reduction ratio of AUC (%)) 4 to 10 bright and also produce results have shown. As also shown in the table 4 and 10, glimepiride (10 mg/kg) alone using compounds that are produced in embodiments 9 not to induce (30 mg/kg, or 100 mg/kg) administered in combination than as in the picomolar excellent hypoglycemic effect. Thus, according to the invention novel 3 - (4 - (benzyloxy) phenyl) - 4 - [heyk[heyk] inosinate wing acid derivative is sulfonylureas (Sulfonylurea) class of drugs in combination with rehmanniae, administration of the drugs alone better hypoglycemic effect, the derivatives and other active ingredient pharmaceutical composition comprises obesity, diabetes of type I, diabetes type II, use my party, treating insulin resistance, hyperglycemia, aims, high the Holy Land bloodletting symptoms, hypercholesterolemia or mixed dyslipidemia, hypertriglyceridemia, such as sick X can be useful in the prevention or treatment of metabolic disorders. <Experiment example 10>tooth it will doze [tin[tin] D it comes (Thiazolidinediones, TZD) Series combination medicaments(Oral Glucose Tolerance Test, OGTT) load by administration per oral The novel 3 - (4 - (benzyloxy) phenyl) - 4 - (Thiazolidinediones, TZD) tooth it will doze [tin[tin] D it comes[heyk[heyk] inosinate wing acid derivatives represented by the formula for evaluating the administration of a combination drug sequence which code such as blood glucose in vivo when conducting experiments. 29 to 30 male week zero dietary obesity model after 16 - 18 to a nanoparticulate military North Korean Workers Party mouse embryo (Diet a-Induced Obesity, DIO) 5 each time by separating compounds that are produced in embodiments 9 randomly group (solvent administration volume: 10 ml/kg) was administered orally to each 10 - 30 mg/kg capacity. The, the nothing control army 0. 5%, a comparable car diplopia methyl cellulose (CarboxyMethyl Celluluse, CMC) was administered orally. Also, tooth it will doze [tin[tin] D it comes (Thiazolidinediones, TZD) based drug rosiglitazone (Rosiglitazone) pioglitazone (Pioglitazone) alone is well known and a 10 mg/kg was administered. Further, pioglitazone and rosiglitazone (Rosiglitazone) (Pioglitazone) compounds that are produced 10 - 30 mg/kg was administered 10 mg/kg and embodiments 9 each in combination. The salt water (saline) and test materials to 5 ml/kg was administered orally. 60 minutes, glucose (4 g/kg) was administered orally in an amount of 5 ml/kg. Blood glucose is measured using oh [khwu[khwu] - check active strip (Accu-a chek active stript, Roche diagnostic co.) aeration chamber, measuring time is measured reference - 60, 0, 20, 40, 60 and 120 have puncture pulse crude component glucose administration, blood glucose AUC (Reduction ratio of AUC (%)) 5 to 11 bright and strong rate also produce results have shown. As also shown in the table 5 and 11, rosiglitazone (5 mg/kg) alone not to induce embodiments using compounds that are produced in 9 (10 mg/kg, or 30 mg/kg) administered in combination than as in the picomolar excellent hypoglycemic effect. The pioglitazone (10 mg/kg) alone not to induce embodiments using compounds that are produced in 9 (10 mg/kg) also by performing a hypoglycemic effect when administered combination as in the picomolar. Thus, according to the invention novel 3 - (4 - (benzyloxy) phenyl) - 4 - (Thiazolidinediones, TZD) a tooth it will doze [tin[tin] D it comes[heyk[heyk] inosinate wing acid derivatives in combination with rehmanniae class of drugs, the drug are better hypoglycemic effect when administered alone, the derivatives and other active ingredient pharmaceutical composition comprises obesity, diabetes of type I, diabetes type II, use my party, treating insulin resistance, hyperglycaemia, aims, high the Holy Land bloodletting symptoms, hypercholesterolemia or mixed dyslipidemia, hypertriglyceridemia, such as sick X can be useful in the prevention or treatment of metabolic disorders. <Experiment example 11>-pharmaceutical (Biguanide) Sequence by combined administration oral medicaments(Oral Gluc ose Tolerance Test, OGTT) load per The novel 3 - (4 - (benzyloxy) phenyl) - 4 - (Biguanide)-pharmaceutical [heyk[heyk] inosinate wing acid derivatives represented by the formula for evaluating the administration of a combination drug sequence which code such as blood glucose in vivo when conducting experiments. 11 - 1. Diabetes of type II Detecting BAX(Rat) model experiment 8 week zerothe [cyu[cyu] it is big excessive model (Zucker Diabetic Fatty, ZDF) 16 - 18 after a time detecting BAX male diabetic fat by separating each military North Korean Workers Party randomly group 9 embodiments 5 to a nanoparticulate embryo in each 1 - 10 mg/kg (administered solvent volume: 5 ml/kg) was administered orally to capacity compounds that are produced. The, the vehicle (Vehicle) nothing control army (0. 5%, car diplopia methyl cellulose (CarboxyMethyl Celluluse, CMC)) a comparable oral administered. Also, bi-pharmaceutical drug metformin alone (Metformin) (Biguanide) sequence is well known a 50 mg/kg was administered. Further, 1 - 10 mg/kg to 50 mg/kg and embodiments 9 metformin (Metformin) compounds that are produced in the combination was administered. 60 minutes, glucose (4 g/kg) was administered orally in an amount of 5 ml/kg. Blood glucose is measured using oh [khwu[khwu] - check active strip (Accu-a chek active stript, Roche diagnostic co.) a transparent conductive layer, the time required to measure glucose administration reference - 60, 0, 20, 40, 60 and 120 have strong rate measured blood glucose AUC puncture pulse crude component (Reduction ratio of AUC (%)) 6 to 12 and bright also produce results have shown. Also 6 and the table 12As shown, metformin (50 mg/kg) alone not to induce embodiments using compounds that are produced in 9 (1 mg/kg, 3 mg/kg or 10 mg/kg) administered in combination than as in the picomolar excellent hypoglycemic effect. 11 - 2. SD Detecting BAX(Rat) model experiment 8 - 10 male SD (Sprague Dawley) 7 for detecting BAX week zero minimum using the finger of time are provided to health after OGTT test between individual by using only implementation. 16 - 18 to a nanoparticulate military North Korean Workers Party embryo after one time after the separation of the randomly group 6, vehicle (Vehicle) (0. 5%, car diplopia methyl cellulose (CarboxyMethyl Celluluse, CMC)) or 9 embodiments compounds of the 3 mg/kg, administered metformin (Metformin) have the capacity 10, 50, 100 mg/kg, 3 mg/kg capacity (Metformin) 9 embodiments compounds of metformin combined administration group combination each capacitance 10, 50, 100 mg/kg was administered. The compounds of the 9 to 10 ml/kg was administered oral expense it will grow with embodiments. Vehicle or compounds of embodiments 9 30 minutes after glucose administration (4 g/kg) was administered orally in an amount of 5 ml/kg. The Accu-a chek active strip (Roche diagnostic co.) for measuring when the blood glucose, glucose administration measuring time is measured reference - 30, 0, 20, 40, 60 and 120 have puncture pulse crude component, Also the result of measurement of blood glucose AUC (Reduction ratio of AUC (%)) 8 to 13 bright and strong rate shown. (Mean ± SE) is precursor to experimental characteristic average standard error, differences between the band group regulated significance assays can 'GraphPad Prism 4' software (Graphpad co. , La Jolla, CA, USA) through a ring of one a-way ANOVA (a Dunnett method), p<0. 05 statistically significant difference when the number of passes is runner out. 9 compound when the administration of a combination of the drug at the the maul [thu[thu] gun it pushed with embodiments, environmentally experiment may be observed and source of the AUC than have strong near value sources, each test group in vehicle about 3. 9 - 20. AUC (area under curve) showed strong effect of 2%. This compound when the administration of a combination drug-pharmaceutical embodiments 9 (Biguanide) sequence, Ru-based pharmaceutical drug efficacy can be maximizes a table by a goniophotometer. Thus, according to the invention novel 3 - (4 - (benzyloxy) phenyl) - 4 - (Biguanide) indoor pharmaceutical [heyk[heyk] inosinate wing acid derivatives in combination with rehmanniae class of drugs, the drug are better hypoglycemic effect when administered alone, the derivatives and other active ingredient pharmaceutical composition comprises obesity, diabetes of type I, diabetes type II, use my party, treating insulin resistance, hyperglycaemia, aims, high the Holy Land bloodletting symptoms, hypercholesterolemia or mixed dyslipidemia, hypertriglyceridemia, such as sick X can be useful in the prevention or treatment of metabolic disorders. <Experiment example 12>SGLT2 (sodium/glucose Cotransportor 2) inhibitor-based medicaments By combined administration (Oral Gluc ose Tolerance Test, OGTT) load per oral The novel 3 - (4 - (benzyloxy) phenyl) - 4 - (sodium/glucose cotransportor 2) SGLT2 [heyk[heyk] inosinate wing acid derivatives represented by the formula for evaluating the administration of a combination drug-based inhibitors which code such as blood glucose in vivo when conducting experiments. 8 - 10 male SD (Sprague Dawley) 7 for detecting BAX week zero minimum using the finger of time are provided to health after OGTT test between individual by using only implementation. 16 - 18 to a nanoparticulate military North Korean Workers Party embryo after one time after the separation of the randomly group 6, vehicle (Vehicle) (0. 5%, car diplopia methyl cellulose (CarboxyMethyl Celluluse, CMC)) or 9 embodiments compounds of the 3 mg/kg, emphasis glycol 1, 3, 10 mg/kg is administered (Empagliflozin) capacity have ripple rosin, rosin (Empagliflozin) 1, 3, 10 mg/kg 3 mg/kg capacity 9 embodiments compounds of combined administration group was administered combination each capacitance ripple glycol emphasis. The compounds of the 9 to 10 ml/kg was administered oral expense it will grow with embodiments. Vehicle or compounds of embodiments 9 30 minutes after glucose administration (4 g/kg) was administered orally in an amount of 5 ml/kg. The Accu-a chek active strip (Roche diagnostic co.) for measuring when the blood glucose, glucose administration measuring time is measured reference - 30, 0, 20, 40, 60 and 120 have puncture pulse crude component, Blood glucose AUC (Reduction ratio of AUC (%)) to the measured results have shown strong rate table 14 and also 9. (Mean ± SE) is precursor to experimental characteristic average standard error, differences between the band group regulated significance assays can 'GraphPad Prism 4' software (Graphpad co. , La Jolla, CA, USA) through a ring of one a-way ANOVA (a Dunnett method), p<0. 05 statistically significant difference when the number of passes is runner out. 9 compound when the administration of a combination of the drug at the emphasis glycol ripple rosin and embodiments, the AUC is environmentally experiments are observed near strong sources have higher than threshold value, about 6 vehicle in each test group. 5 - 36. AUC (area under curve) showed strong effect of 6%. This compound when the administration of a combination SGLT2 inhibitor based drugs 9 embodiments, SGLT2 inhibitor sequence table and maximizes the amount of drug efficacy can be by a goniophotometer. Thus, according to the invention novel 3 - (4 - (benzyloxy) phenyl) - 4 - wing acid derivative is SGLT2 [heyk[heyk] inosinate (sodium/glucose cotransportor 2) inhibitors in combination with rehmanniae class of drugs, the drug are better hypoglycemic effect when administered alone, the derivatives and other active ingredient pharmaceutical composition comprises obesity, diabetes of type I, diabetes type II, use my party, treating insulin resistance, hyperglycaemia, aims, high the Holy Land bloodletting symptoms, hypercholesterolemia or mixed dyslipidemia, hypertriglyceridemia, such as sick X can be useful in the prevention or treatment of metabolic disorders. <Experiment example 13>GLP-a 1 secretion analysis experiments NCI a-H716 cells 12 well plate coated mat gel 1X10 (BD)6 Cells/well (cells/well) to 48 after seeding time incubator (37 °C) was cultured. Supernatant after low glucose DMEM (low glucose) (5. 5 mm; 2% FBS, 2 mm L - glutamine, penicillin-resistant Streptococcus feed containing 100 IU/ml and 100 ug/ml) the surfactant is non-nutritive medium was cut into 4 time to supply (starvation). Supernatant after at the time of hit writing [lip[lip] (0. 1, 1, or 10 μm) DMEM (high glucose) diluted high glucose (25 mm) after 30 minutes pretreatment medium was replaced. 30 minutes 9 embodiments each compound concentration (1, 10, or 30 μm) in 37 °C after treated with him as time 2. 0 entering the contrast. 1% DMSO using the air. With the experiment using the supernatant cells (Millipore) GLP-a 1 (Glucagon-a Like Peptide-a 1) kit to NCI a-H716 secreted GLP-a 1 in amount were measured (table 15 and also 10 reference). As a result at the time of hit writing [lip[lip]at the time of hit writing [lip[lip] and embodiments 9 the method is a combination of the group consisting of GLP-a 1 alone processing than the compounds by a significant increase in the observed. <Experiment example 14>insulin secretion experiments The 24 well plate for detecting BAX (Insulinoma) cell lines derived from insulin species 5X10 INS-a 1 cells5 Cells/well (cells/well) by culturing 48 time to work through. 3 mm glucose - KRB buffer (118 mm NaCl, 4. 7 mm KCl, 1. 2 mm KH2 PO4 , 1. 16 mm MgCl2 , 10 mm HEPES, 2. 5 mm CaCl2 , 25. 5 mm NaHCO3, 0. 2% BSA, pH 7. 4) culturing cells such as cells from a post-wash 2 buffer intracellular glucose concentration in low-concentration state time to work through. The final concentration of test compound (table 16 reference) 25 mm glucose - KRB buffer 0. 1 - 10 M is then diluted to 3 mm glucose induced insulin secretion by treating the plant cells 1 are replaced with time. With the experiment using insulin ELISA kit (Morinaga) secreted into the supernatant cells amount were measured (table 16 and also 11 reference) insulin. As a result writing it cuts the lama id which will grow and embodiments 9 compounds in combination of embodiments 9 has been confirmed that the increase of insulin secretion than [thwu[thwu] woman army[thwu[thwu] woman army compound alone. <Formulation example 1>pharmaceutical manufacturing 1 - 1. it buys proposal Manufacturing Compounds of formula 1 500 mg 100 mg lactose- 10 mg talc The above components are mixed to prepare a filled with an airtight formed thereon can be disintegrated. 1 - 2. Tablet manufacturing Compounds of formula 1 500 mg 100 mg corn starch 100 mg lactose- Magnesium salt of stearic acid 2 mg The conventional process for preparing tablets the tablets after mixing components by a lubricant homogeneously to prepare. 1 - 3. For the preparation of capsules Compounds of formula 1 500 mg 100 mg corn starch 100 mg lactose- Magnesium salt of stearic acid 2 mg According to the conventional capsule making method of mixed before or gelatin capsule to prepare a capsule agent. *1 - 4. The steps of Compounds of formula 1 500 mg Sterile distilled water for injection dosage PH modulators returned According to conventional steps of 1 per ampoule (2 ml) to prepare the component content. 1 - 5. These Manufacturing Compounds of formula 1 100 mg 10 g per isomerization 5 g mannitol Purified returned These purified water by applying conventional dissolving each component is formed by crystallizing lemon aroma components may be mixed together and then applied to the entire value of the dosage of 100 ml brown bottle after adjusting the total treated water treated water applied for feeding liquid to prepare a sterile filling. In specific embodiments the invention more precisely techniques that are directionally-bar portion, having conventional knowledge to the party industry traded only in such preferred yale is received, the ms. point is not adjusting the scope of this invention. Thus, a substantial range of equivalent with clause defined by attached claims that will. The present invention relates to a pharmaceutical composition for preventing or treating metabolic diseases, in which a novel 3-(4-(benzyloxy)phenyl)hex-4-inoic acid derivative and at least another active ingredient, which is selected from the group consisting of dipeptidyl peptidase-4 (DPPIV) inhibitor-based, sulfonylurea-based, thiazolidinedione (TZD)-based, biguanide-based, and sodium/glucose cotransporter 2 (SGLT2) inhibitor-based drugs, may be administered in combination or in the form of a composite preparation. The use of the composition of the present invention can provide a remarkably excellent blood sugar reducing effect in various animal diabetic disease models, and the composition of the present invention can be favorably used as a pharmaceutical composition for preventing or treating metabolic diseases, such as obesity, diabetes type I, diabetes type II, glucose intolerance symptoms, insulin resistance symptoms, hyperglycemia, hyperlipidemia, hypertriglyceridemia, hypercholesterolemia, dyslipidemia, and syndrome X. (A) compounds of formula 1 as effective first 1, their isomers, hydrates, solvates or pharmaceutically acceptable salts thereof and (b) 2 (Dipeptidyl peptidase-a 4) inhibitors as first effective d [pheyp[pheyp] mote [til[til] mote day [cu[cu] IV family, sulfonylureas (Sulfonylurea) sequence, tooth it will doze [tin[tin] D it comes (Thiazolidinediones) sequence, and SGLT2 inhibitor compounds (sodium/glucose cotransporter 2) (Biguanide) sequence-pharmaceutical consisting of one or more compounds selected from the group consisting of obesity, diabetes of type I, diabetes type II, use my party, treating insulin resistance, hyperglycemia, aims, high the Holy Land bloodletting symptoms, hypercholesterolemia or mixed dyslipidemia, hypertriglyceridemia and composition for the prevention or treatment of metabolic disorders selected from the group consisting X syndrome: [formula 1] (In the formula 1, A single bond, or a double bond and; A and E are independently C, or N and; and n is 0 - 1; X is single bond, or a straight or branched alkyl [leyn[leyn] And C1 provided 3; R1 Is - H, or And; R2 R is either H or3 Phenyl can be linked together to form a; R3 Is either H, R2 Forming a connected with phenyl, or R4A Linked together form a substituted or non-substituted phenyl can be maul [thok[thok] town; R4A Is - H, - OH, =O, , , , , , , , , , , , Or Either, or R3 Linked together form a substituted or non-substituted phenyl can be maul [thok[thok] town; R4B The member or, or R4B Connected atom and R4A With Can be formed; and R5 H is are disclosed.) Deletion Deletion According to Claim 1, compounds of the formula 1 is a compound selected from the group consisting or a composition comprising either: (1) 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - tooth [heyk[heyk] inosinate; (2) 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - L - inosinate [heyk[heyk] tooth lysine salt; (3) 4 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - tooth [heyk[heyk] inosinate; (4) 3 - (4 - (3 - (4 - oxo - 1 - enyl gap claw [heyk[heyk]) benzyloxy) phenyl) inosinate [heyk[heyk] 999000 7468999 tooth; (5) 3 - (4 - (3 - (4 - hydroxy - 1 - enyl current events claw [heyk[heyk]) benzyloxy) phenyl) - 4 - tooth [heyk[heyk] inosinate; (6) 3 - (4 - (3 - (4 - hydroxy - 1 - enyl current events claw [heyk[heyk]) benzyloxy) phenyl) - 4 - L - inosinate [heyk[heyk] tooth lysine salt; (7) (3S) - 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - tooth [heyk[heyk] inosinate; (8) (3R) - 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - tooth [heyk[heyk] inosinate; (9) (3S) - 3 - (9990007534 999 (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - L - inosinate [heyk[heyk] tooth lysine salt; (10) (3R) - 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - tooth [heyk[heyk] inosinate L - lysine salt; (11) (3S) - 3 - (4 - (3 - (1, 4 - [4, 5] - 7 - en - 8 - oxa cyber to die process for one) benzyloxy) phenyl) - 4 - tooth [heyk[heyk] inosinate sodium salt; (12) 3 - (4 - (4 - ((3, 4 - dihydroisoquinoline -2 (1H) - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (13) 3 - (4 - (3 - cyclo [...][...] ((3, 4 - (1H) indoline -2 d high draw cow [khwi[khwi] glow - yl) methyl) - 4 - benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (14) 3 - (4 - (4 - ((4 - phenyl - 5, 6 - dihydropyridine -1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (15) 3 - (4 - (4 - ((4 - phenyl piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (16) 3 - (4 - (4 - ((6 - methoxy - 3, 4 - dihydroisoquinoline -2 (1H) - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (17) 3 - (4 - (4 - ((4 - phenylpiperidine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (18) 3 - (4 - (4 - ((4 - (4 - fluorophenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (19) 3 - (4 - (4 - ((4 - (4 - (trifluoromethyl) phenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (20) 3 - (4 - (4 - ((4 - (4 - (3 - (methylsulfonyl) propoxy) phenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (21) (S)- 3 - (4 - (4 - ((3, 4 - dihydroisoquinoline -2 (1H) - yl) methyl) benzyloxy) phenyl) - 4 - (22) wing [eyk[eyk] Hour [tu[tu] 999 0010465999 [heyk[heyk] inosinate (S)- 3 - (4 - (4 - ((4 - (4 - (trifluoromethyl) phenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (23) (S)- 3 - (4 - (4 - ((4 - (4 - fluorophenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (24) potassium (S)- 3 - (4 - (4 - ((4 - (4 - (trifluoromethyl) phenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate; (25) (S)- 3 - (4 - (4 - ((6 - methoxy - 3, 4 - dihydroisoquinoline -2 (1H) - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (26) (S)- 3 - (4 - (4 - ((4 - phenylpiperidine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (27) (S)- 3 - (4 - (4 - (- 2 - ylmethyl isoindoline) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (28) (S)- 3 - (4 - (4 - ((4 - phenyl - 5, 6 - dihydropyridine -1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (29) (S)- 3 - (4 - (4 - ((4 - (4 - (maul [thok[thok] hour maul [thok[thok] city) phenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) [heyk[heyk] 9990007 861999 [eyk[eyk] Hour [tu[tu] inosinate wing; (30) (S)- 3 - (4 - (4 - ((4 - (5 - isopropyl - 1, 2, 4 - oxadiazole - 3 - yl) piperidine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (31) (S)- 3 - (4 - (4 - ((4 - (5 - isopropyl - 1, 2, 4 - oxadiazole - 3 - yl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (32) (S)- 3 - (4 - (4 - ((4 - (4 - (methylsulfonyl) phenyl) - 5, 6 - dihydropyridine -1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (33) (S)- 3 - (9990 007926999 (4 - ((4 - (4 - (3 - (methylsulfonyl) propoxy) phenyl) - 5, 6 - dihydropyridine -1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (34) (3S) - 3 - (4 - (4 - (1 - (3, 4 - dihydroisoquinoline -2 (1H) - yl) ethyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (35) (S)- 3 - (4 - (4 - ((4 - (4 - hydroxyphenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (36) (S)- 3 - (4 - (4 - ((4 - (4 - (3 - (methylsulfonyl) propoxy) phenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (37) sodium (S)- 3 - (4 - (4 - (- 2 - isoindoline ylmethyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate; (38) L - lysine (S)- 3 - (4 - (4 - (- 2 - isoindoline ylmethyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate; (39) (S)- 3 - (4 - (4 - ((4 - (4 - fluorophenyl) - 5, 6 - dihydropyridine -1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (40) (S)- 3 - (4 - (4 - ((4 - (4 - methoxyphenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) 999 0008048999 [heyk[heyk] inosinate wing [eyk[eyk] Hour [tu[tu]; (41) sodium (S)- 3 - (4 - (4 - ((3, 4 - dihydroquinoline -1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate; (42) potassium (S)- 3 - (4 - (4 - ((3, 4 - dihydroquinoline -1 (2H) - yl) methyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate; (43) (S)- 3 - (4 - (4 - ((4 - (benzo thiazol - 2 - yl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (44) (S)- 3 - (4 - (4 - ((4 - (5 - propyl pyrimidine - 2 - yl) piperazine 9990 008112999 yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (45) (S)- 3 - (4 - (4 - ((4 - (5 - cyano - 2 - yl roh blood [tin[tin]) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (46) (3S) - 3 - (4 - (4 - ((3 - phenylpyrrolidin - 1 - yl) methyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (47) sodium (S)- 3 - (4 - (4 - ((4 - (4 - methoxyphenyl) piperazine - 1 - yl) methyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate; (48) (S)- 3 - (4 - (4 - (2 - (6 - methoxy - 3, 4 - dihydroisoquinoline -2 (1H) - yl) ethyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (49) (S)- 3 - (4 - (4 - (2 - (isoindolin - 2 - yl) ethyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; (50) (S)- 3 - (4 - (4 - (2 - (3, 4 - dihydroisoquinoline -2 (1H) - yl) ethyl) benzyloxy) phenyl) - 4 - wing [eyk[eyk] Hour [tu[tu][heyk[heyk] inosinate; and (51) sodium (S)- 3 - (4 - (4 - ((6 - methoxy - 3, 4 - dihydroisoquinoline -2 (1H) - yl) methyl) benzyloxy) phenyl) - 4 - hydroxycyclohexyl [heyk[heyk] inosinate. According to Claim 1, IV inhibitor sequence is the d [pheyp[pheyp] mote [til[til] mote day [cu[cu]at the time of hit writing [lip[lip] (Sitagliptin), billets it will bring near, [lip[lip] (Vildagliptin), [sak[sak] It buys the writing [lip[lip] (Saxagliptin), contamination in writing [lip[lip] (Linagliptin), reel four writing [lip[lip] (Teneligliptin), with egg writing [lip[lip] (Alogliptin), my Americawriting [lip[lip] (Gemigliptin), two toggles [lip[lip] (Dutogliptin), base (Berberine), loupe this year (Lupeol), selected from the group consisting of coffee (dandelion coffee) and red (red alder) [ayl[ayl] Compared toit is burnt it comes only cost characterized composition. According to Claim 1, polymerization of the sulfonylureas sequence is polyimide (Carbutamide) carboxylic acid, butenoic [heyk[heyk] It buys America [tu[tu] (Acetohexamide), claw [lu[lu] pro group America [tu[tu] (Chlorpropamide), [thol[thol] Father hit America [tu[tu] (Tolbutamide), writing pizza id (Glipizide), gliclazide (Gliclazide), writing it cuts the lama id which will grow (Glibenclamide), writing step [lu[lu] world [tu[tu] (Glibornuride), writing [khwi[khwi] money (Gliquidone), writing small storms of life id (Glisoxepide), writing nose bleed America [tu[tu] (Glyclopyramide) and glimepiride (Glimepiride) selected from the group consisting of cost characterized composition. According to Claim 1, the tooth it will doze [tin[tin] D it comes sequence is (Rosiglitazone) rosiglitazone, pioglitazone (Pioglitazone), of preventing (Troglitazone), four toggle it burns the zone (Netoglitazone), rosiglitazone (Rivoglitazone) ribo, selected from the group consisting of rhodanine and hour writing it burns the zone (Ciglitazone) (Rhodanine) cost characterized composition. According to Claim 1, holds the pharmaceutical sequence is metformin (Metformin), the pen gun it pushed (Phenformin), the vice-pawl it pushed (Buformin), pro it will not be nine (Proguanil), claw [lu[lu] pro nine will not be (Chlorproguanil), chlorhexidine (Chlorhexidine), polyamino profile pharmaceutical (Polyaminopropyl biguanide (PAPB)), poly [heyk[heyk] San'A idthe rack which will knowat the time of [tin[tin] (Alexidine) selected from the group consisting (Polihexanide) and cost characterized composition. According to Claim 1, the SGLT2 inhibitor compounds is emphasis glycol ripple rosin (empagliflozin), selected from the group consisting (dapagliflozin) (canagliflozin) and multi green onion writing ripple rosincar orwriting ripple rosin cost characterized composition. According to Claim 1, 2 1 effective the blending of the substances and the effective weight ratio 0. Compositions characterized in 03:1 to 100:1. According to Claim 1, the composition GPR40 (G-a protein receptor 40) activating enzymes characterized pharmaceutical compositions for the prevention or treatment of metabolic disorders. DeletionEmbodiments Chemical structure Embodiments Chemical structure 1 27 2 28 3 29 4 30 5 31 6 32 7 33 8 34 9 35 10 36 11 37 12 38 13 39 14 40 15 41 16 42 17 43 18 44 19 45 20 46 21 47 22 48 23 49 24 50 25 51 26 Embodiments EC50 (ΜM) 1 C 2 C 3 C 4 C 5 B 6 B 7 A 8 C 9 A 10 C 11 A 12 A 13 C 14 A 15 C 16 C 17 C 18 C 19 C 20 C 21 B 22 C 23 B 24 B 25 C 26 C 27 A 28 A 29 B 30 C 31 C 32 C 33 C 34 C 35 C 36 C 37 A 38 A 39 B 40 B 41 C 42 C 43 C 44 C 45 C 46 C 47 B 48 C 49 C 50 C 51 C Comparison example 1 B Comparison example 2 C Compound EC expected50 Efficacy value Embodiments 9 The measurable concentration (1 nm) the lower parts of the Comparison example 1 14 nm Comparison example 3 27 nm Embodiments (10 μm) CYP inhibition (%) 1A 2 2C 9 2C 19 2D 6 3A 4 1 0 42. 8 18. 3 1. 9 12. 7 3 0 21. 1 19. 4 6. 0 33. 1 4 0 41. 5 45. 4 19. 3 35. 0 7 4. 3 47. 1 3. 7 13. 9 15. 5 9 4. 3 47. 1 3. 7 13. 9 15. 5 21 4. 0 75. 9 46. 5 16. 1 27. 3 26 0. 7 31. 5 13. 2 2. 3 14. 1 29 0. 7 26. 7 9. 7 18. 2 0 36 16. 6 0 10. 8 1. 8 11. 5 38 2. 2 34. 4 13. 2 15. 6 18. 1 40 9. 7 18. 4 19. 5 17. 9 0 Comparison example 1 0. 8 81. 2 12. 4 4. 3 10. 0 Comparison example 2 0 43. 9 34. 5 63. 2 42. 0 Embodiments % AUC 2 17. 2 3 12. 5 4 16. 2 6 15. 2 9 24. 7 12 31. 0 14 27. 7 16 21. 1 25 24. 6 29 27. 1 36 22. 6 37 28. 5 41 23. 7 43 21. 2 44 22. 8 Comparison example 1 16. 2 Embodiments Dose (mg/kg) % AUC 5 embodiments 1 B 3 B 10 A Embodiments 9 1 B 3 A 10 A 14 embodiments 1 C 3 B 10 B 28 embodiments 1 B 3 B 10 B Embodiments 37 1 A 3 A 10 A 47 embodiments 1 C 3 C 10 B Comparison example 1 10 B Compound Blood glucose AUC drop rate (%) at the time of hit writing [lip[lip] (10 mg/kg) 17. 7 9 embodiments (30 mg/kg) 10. 1 Embodiments 9 (100 mg/kg) 15. 4 at the time of hit writing [lip[lip] (10 mg/kg) (30 mg/kg) + 9 embodiments 20. 2 at the time of hit writing [lip[lip] (10 mg/kg) (100 mg/kg) + 9 embodiments 26. 2 Compound Blood glucose AUC drop rate (%) 9 embodiments (3 mg/kg) 14. 8 (1 mg/kg) writing [lip[lip] contamination in 5. 8 (3 mg/kg) writing [lip[lip] contamination in 7. 9 (10 mg/kg) writing [lip[lip] contamination in 11. 8 9 embodiments (3 mg/kg) (1 mg/kg) + writing [lip[lip] contamination in 24. 1 9 embodiments (3 mg/kg) (3 mg/kg) + writing [lip[lip] contamination in 24. 3 9 embodiments (3 mg/kg) (10 mg/kg) + writing [lip[lip] contamination in 24. 4 Compound Blood glucose AUC drop rate (%) Glimepiride (10 mg/kg) 44. 6 9 (10 mg/kg) embodiments 9. 1 9 embodiments (30 mg/kg) 11. 4 Embodiments 9 (100 mg/kg) 12. 7 Glimepiride (10 mg/kg) (10 mg/kg) + 9 embodiments 49. 6 Glimepiride (10 mg/kg) (30 mg/kg) + 9 embodiments 51. 6 Glimepiride (10 mg/kg) (100 mg/kg) + 9 embodiments 53. 9 Compound Blood glucose AUC drop rate (%) Rosiglitazone (5 mg/kg) 17. 1 Pioglitazone (10 mg/kg) 27. 9 9 (10 mg/kg) embodiments 24. 9 9 embodiments (30 mg/kg) 20. 2 Rosiglitazone (5 mg/kg) (10 mg/kg) + 9 embodiments 23. 5 Rosiglitazone (5 mg/kg) (30 mg/kg) + 9 embodiments 23. 3 Pioglitazone (10 mg/kg) (10 mg/kg) + 9 embodiments 29. 2 Pioglitazone (10 mg/kg) (30 mg/kg) + 9 embodiments 27. 2 Compound Blood glucose AUC drop rate (%) Metformin (50 mg/kg) 21. 7 9 embodiments (1 mg/kg) 34. 2 9 embodiments (3 mg/kg) 40. 9 9 (10 mg/kg) embodiments 37. 8 Metformin (50 mg/kg) (1 mg/kg) + 9 embodiments 43. 0 Metformin (50 mg/kg) (3 mg/kg) + 9 embodiments 48. 8 Metformin (50 mg/kg) (10 mg/kg) + 9 embodiments 48. 3 Compound Blood glucose AUC drop rate (%) 9 embodiments (3 mg/kg) 14. 5 Metformin (10 mg/kg) 3. 9 Metformin (50 mg/kg) 7. 3 Metformin (100 mg/kg) 10. 0 9 embodiments (3 mg/kg) (10 mg/kg) + metformin 20. 2 9 embodiments (3 mg/kg) (50 mg/kg) + metformin 16. 5 9 embodiments (3 mg/kg) + metformin (100 mg/kg) 18. 7 Compound Blood glucose AUC drop rate (%) 9 embodiments (3 mg/kg) 14. 8 Emphasis (1 mg/kg) glycol ripple rosin 6. 5 Emphasis (3 mg/kg) glycol ripple rosin 9. 2 Emphasis glycol (10 mg/kg) ripple rosin 29. 5 9 embodiments (3 mg/kg) (1 mg/kg) + emphasis glycol ripple rosin 29. 3 9 embodiments (3 mg/kg) (3 mg/kg) + emphasis glycol ripple rosin 30. 2 9 embodiments (3 mg/kg) (10 mg/kg) + emphasis glycol ripple rosin 36. 6 Compound Absorbing member relative ratio (%) for matching each experimental group GLP-a 1 secretion Matching opening 100. 0 ± 2. 3 at the time of hit writing [lip[lip] (0. 1 μm) 127. 5 ± 7. 3 at the time of hit writing [lip[lip] (0. 1 μm) (10 μm) + 9 embodiments 155. 7 ± 3. 4 at the time of hit writing [lip[lip] (0. 1 μm) (30 μm) + 9 embodiments 219. 6 ± 3. 7*** (1 μm) at the time of hit writing [lip[lip] 128. 1 ± 0. 6 at the time of hit writing [lip[lip] (1 μm) (10 μm) + 9 embodiments 185. 7 ± 2. 0### at the time of hit writing [lip[lip] (1 μm) (30 μm) + 9 embodiments 241. 4 ± 2. 0### at the time of hit writing [lip[lip] (10 μm) 184. 9 ± 0. 2 at the time of hit writing [lip[lip] (10 μm) (10 μm) + 9 embodiments 216. 9 ± 6. 7 at the time of hit writing [lip[lip] (10 μm) (30 μm) + 9 embodiments 221. 9 ± 6. 0$ Compound Insulin (ng/ml) SEM Vehicle 58 2. 29 writing it cuts the lama id which will grow (0. 1 μm) 73 2. 48 (1 μm) writing it cuts the lama id which will grow 72 1. 51 9 embodiments (0. 01 μm) 99 6. 41 9 embodiments (0. 01 μm) + writing it cuts the lama id which will grow (0. 1 μm) 133 10. 98 9 embodiments (0. 01 μm) (1 μm) + writing it cuts the lama id which will grow 128 9. 14